One of the properties of copper, which has done much to give it its present prominent place among the useful metals, is its electrical conductivity,—a property which has now become the chief criterion of the value of the commercial product. In pursuance of that distinctively American principle that, “ the best is none too good,” metal of the highest conductivity is usually called for even when such extreme purity is of no advantage ; for instance, in the making of brass and other alloys. The average brass-founder feels nervous unless his copper is so very pure that it shows a conductivity of 99 or 100 per cent., while the character of the zinc used is usually overlooked in his anxiety.
Electrolytic refining has made it possible to produce copper of a very high degree of purity, the metallic impurities averaging but a few thousandths of 1 per cent.; oxygen, usually present in the form of cuprous oxide, bringing the total up to about a tenth of 1 per cent. The published data bearing upon the relationship between chemical purity and electrical conductivity is, however, very scant, nearly all the work that has been done having dealt with alloys carrying considerable quantities of the foreign elements. In this paper I have brought together the results of experiments extending over a period of several years, that have been carried out with the idea of determining the amount of various elements which would lower the conductivity 3 or 4 per cent.—a series of impure coppers rather than alloys.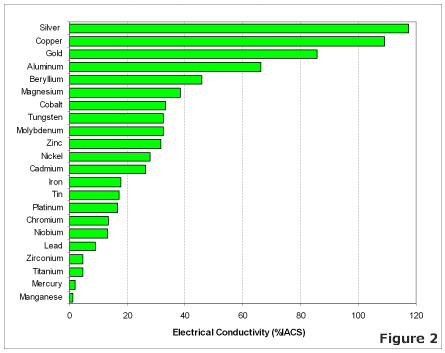
There are many precautions necessary to keep the conditions identical in different experiments, since molten copper is very sensitive chemically to its surroundings and the quantity of many substances required to lower its conductivity several per cent., is exceedingly minute. The method of procedure adopted after considerable experimenting was as follows:
The copper used was in the form of No. 12 B. & S. annealed wire, compacted together and placed in a round Battersea crucible, size H, 500 g. constituting a charge. The impurity was added in one of three ways, depending upon its melting-point,—1. If high, it was dumped in a weighed amount directly into the bottom of the crucible. 2. If comparatively low, some of the copper wire was rolled out into foil and the impurity encased in it, the whole being put at the bottom of the crucible; and 3. If very low (such as phosphorus), it was wrapped in foil as before, but thrust under the surface of the molten copper just before pouring the samples. After the wire was rammed home in the crucible, a layer of broken charcoal about an inch thick was placed on top, the whole filling the crucible. A lid was put on the crucible which was then subjected to a strong blast in a gas-furnace. The sample was poured 25 min. after turning on the blast, which was just sufficient time to melt the charge thoroughly without over-heating it, and not appreciably alter the “ pitch ” of the copper. No trouble was experienced in getting elements much below their melting-points at this heat to dissolve in the molten copper. Castings were made in a heated iron mold in the shape of slightly tapering cylinders having an average diameter of 0.5 in. and a length of 7 inches. These castings were swaged hot on an anvil to 3/8 in. in diameter and then drawn cold to No. 12 B. & S. wire. Then, in order to eliminate the effects of the drawing, the wires were annealed electrically by forcing them to carry a current of 110 amperes. The electric method of annealing is a very simple way of accurately duplicating results, the temperature of annealing having a marked influence on the conductivity. The wires were measured for conductivity and then analyzed for copper and the foreign element. The sum of the copper plus the impurity was a general check on the analysis and the “ pitch.”
Table I. gives a general summary of the data, the elements being arranged alphabetically. The data are also expressed graphically in Figs. 1 to 16. The alloying elements were obtained in as pure a form as possible, and it is not believed that the results are greatly influenced by impurities in them. These were,—proof-silver 1,000 fine; gold in the form of prills from bullion-analyses carrying no impurity, but a negligible amount of silver; test-lead; electrolytic iron free from carbon; and sulphur as cupric sulphide. The other elements were of the chemically pure (C. P.) grade obtainable in the market.
In examining the results it is natural to group the elements in their order in the periodic system. This is done in Table II. The column headed “ factor ” gives the ratio of the lowering of the conductivity, to the amount of the impurity present. In obtaining this ratio, tangents to the curves in Figs. 1 to 16 have been used, so that the ratios hold strictly true only in the case of an infinitely small lowering. The factor is seen to bear a general relation to the periodic arrangement, decreasing with increasing atomic weight within any one group, though there is no evident relation between one group and another. This factor is of use when examining the analysis of a copper which shows low conductivity, as a means of indicating the probable cause of the trouble. The data do not yield even an approximate constant for molecular lowering of the conductivity—that


is, the “ factor ” multiplied by the atomic weight—even within single periodic groups.
The form of the composition-conductivity curve of a binary alloy may be one of four different types.
- When the constituents are mutually soluble in all propor-










![]()





tions. The composition-conductivity plot of such a case gives a smooth curve throughout.
2. When the constituents are mutually insoluble in any proportions, in which case the conductivity-curve becomes a straight line, the so-called alloy being but a mechanical mixture.
3. When the mutual solubility is limited, there is a soluble series at each end of the curve and a eutectiferous series in the middle. Since two substances absolutely insoluble in one another probably do not exist, case 2 cannot be rigidly applied, such substances forming an extreme application of case 3.
4. Lastly, there are cases where definite chemical compounds are formed, which may give a curve with a soluble series at one end and a wholly eutectiferous series at the other.
The results given in this paper deal entirely with the first very short part of the conductivity-curve, and it therefore should be expected that some curvature would be shown in every case. Some of the elements employed are so nearly insoluble in copper, however, that the curve differs but little from a straight line connecting the conductivities of the two pure substances in the mixture.
The useful alloys of copper comprise mixtures with zinc, tin, aluminum, silicon and phosphorus. Zinc and tin are added in relatively large quantities to form brasses and bronzes. Aluminum, silicon and phosphorus are added in comparatively small quantities and their ability to deoxidize copper that has been needlessly exposed to the air in melting, probably comes much more frequently into play when making castings than the founder imagines. Under proper conditions it is perfectly possible to make copper-castings of perfect soundness and conductivity, without the use of fluxes. But, looking at the table of factors, it is seen that no worse elements could have been picked out from an electrical standpoint than those above mentioned. On the other hand, the elements which have but little effect upon the electrical conductivity—lead, bismuth and tellurium—make the metal extremely brittle. This effect seems to be a question of solubility, the last-named elements being practically insoluble in copper. We then have a mechanical mixture in which the conductivity is only affected in accordance with the proportions of the mixture, while the mechanical properties of the foreign element itself are brought into prominence.
With the other classes of substances a very different state of affairs exists, the alloy being copper embedded in a matrix of an alloy of copper and the impurity. The conductivity of this matrix is, in general, low, and its amount is beyond all proportion to that of the impurity added, and hence the magnified depression of the conductivity of the whole. Metallographic work with the microscope bears this out; 0.1 per cent, of bismuth, for example, causing but a thin skin around the copper crystals, while the same amount of arsenic forms a thick wall. It is often possible to counteract the detrimental mechanical effects of one impurity by adding another, as in the case where a relatively innocuous impurity may dissolve an otherwise insoluble one, like lead.
The results given in Table I. show what a severe requirement the customary 97- or 98-per cent, conductivity-specification is, especially as copper is usually associated with arsenic; and when it is considered that the average electrolytic refinery daily passes this requirement by a margin of 2 or 3 per cent., frequently using anodes containing 1 per cent, or more of arsenic, it will be appreciated that electrolysis would have come as a refining operation even did copper never carry gold or silver.
