Cementation of copper onto iron is a historically old process for recovery of copper from leach solutions. Although about 13 percent of the Nation’s primary copper production is from cementation, this process remains more of an art than a science. The literature on cementation is extensive, but a thorough review shows that until about 1965 the articles published were descriptions of plant practice, surveys and reviews of the art, or reports from relatively crude laboratory studies. About 1965, there was a renewed interest in cementation by the scientific community, and several excellent papers on copper cementation kinetics have been published in the United States, Canada, and Australia.
All of these studies have contributed to the understanding of cementation, but most were done in the absence of a copper deposit on the iron surface or disregarded the effect of deposited copper. Because of this disregard for the effect of deposited copper, statements have often been made that copper deposited on the iron retards the cementation rate, and that a clean iron surface is essential for efficient cementation. A few investigators have recognized the potential of the deposit to enhance the cementation rate, but their observations have only been utilized to explain anomalous experimental results. The revolving drum cementation system used in this study allows some cemented copper to remain attached to the iron precipitant, and results from the kinetic study indicate that the cemented copper attached to the iron actually enhances the reaction rate instead of retarding it.
Cementation Theory
Copper cementation has been shown to be a transport-controlled process by several investigators in carefully selected systems where a copper deposit on the iron surface was absent or minimal Strickland and Lawson were the first investigators to propose that the cementation process occurs as a series of electrochemical cells. A modification of their approach to cementation is shown in figure 1 which illustrates the process after a copper deposit has been established on the iron surface. Cupric ion is visualized to precipitate on growing copper dendrites attached to the iron surface; thus, removal of cemented copper is not necessary for continuous cementation. The large surface area created by the dendritic copper deposit results in an increase in the cementation rate. Although the deposited copper appears to cover the iron surface, the deposit is porous enough to allow
Cu+² + 2e = Cu°
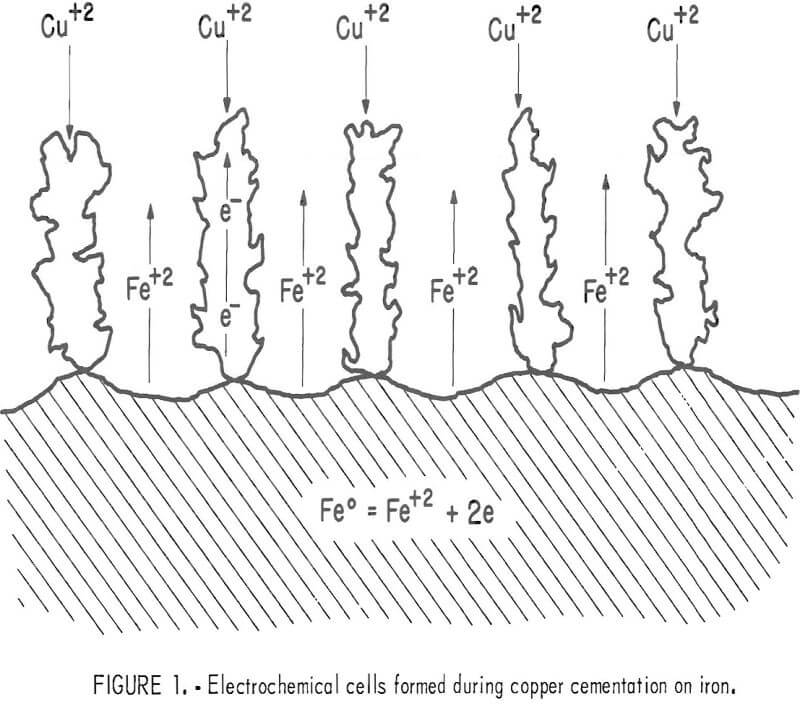
dissolved iron to diffuse away from the surface into the bulk solution. Current views of cementation as expressed in recent publications and patents recommend removal of the copper deposit from the iron by various means. The electrochemical approach to cementation indicates that substantial removal of deposited copper is actually detrimental to the efficiency of cementation.
Experimental Procedure and Apparatus
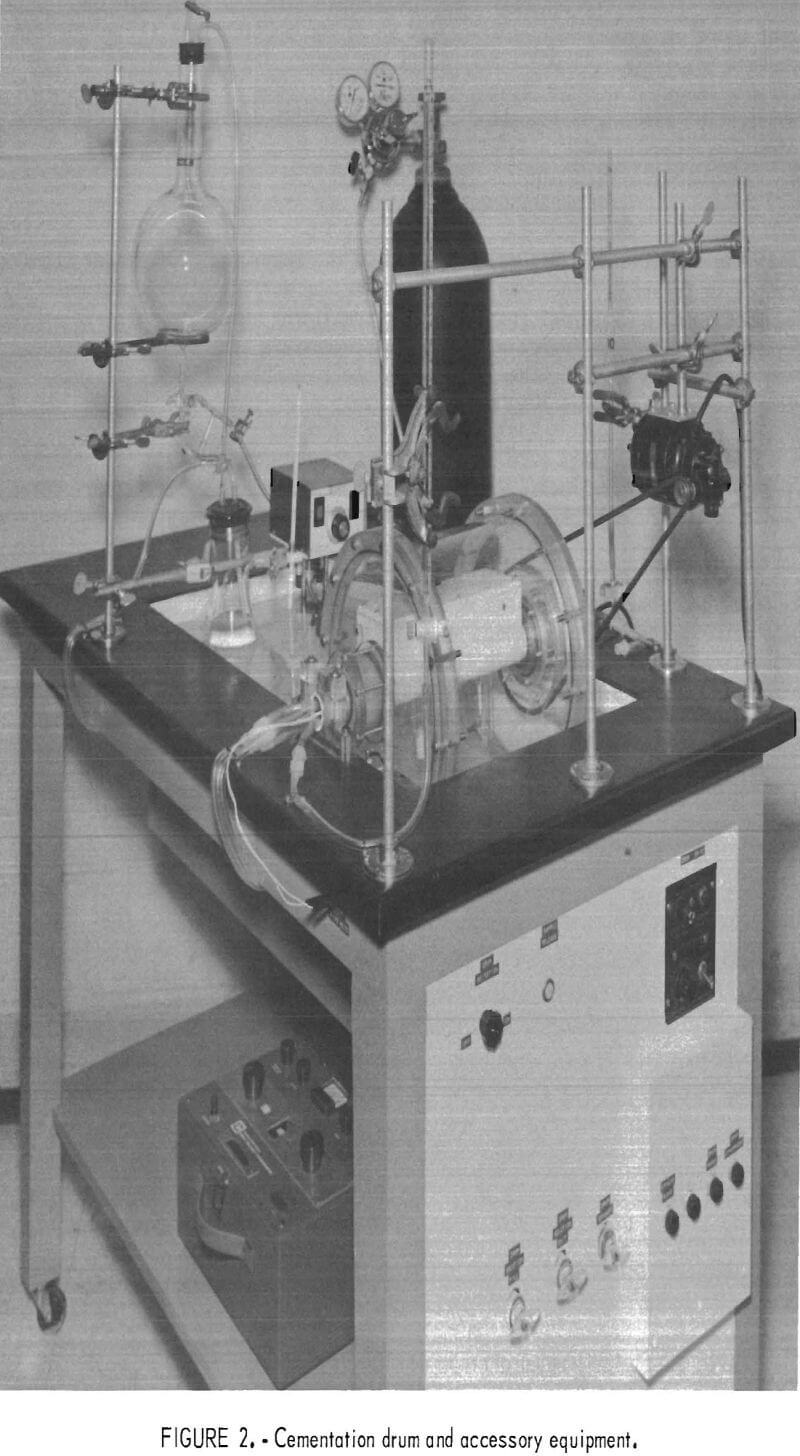
The cementation study was conducted in the revolving drum system illustrated in figure 2. The drum was constructed of acrylic plastic and was equipped with an internal instrument box containing a stirring motor, sampling system, gas-solution inlets and outlets, temperature sensor, pH electrodes, and sample drop system. The drum was partially submerged in a constant-temperature bath to maintain the desired test temperature.
All test solutions were prepared from reagent-grade chemicals, and the iron precipitant for each test was 6-d finishing nails. Analysis for copper and iron was accomplished by atomic absorption spectroscopy.
Tests were carried out using 3.25 liters of solution and 40 nails having a total geometric surface area of 148.8 cm². Prior to each cementation test, the drum, accessory equipment, and solution were purged with nitrogen gas to establish a neutral atmosphere for the test. Solution samples were taken at specified time intervals, diluted, and analyzed for copper and iron content.
Results and Discussion
Several striking results have emerged from this study, and each supports the contention that the cementation process is significantly affected by the copper deposit.
In the first part of this investigation, microscopic examination of cementation on a flat iron surface allowed observation and photography of copper dendrite growth on the iron surface. Figure 3 illustrates the growth of copper around a hydrogen gas bubble on the iron
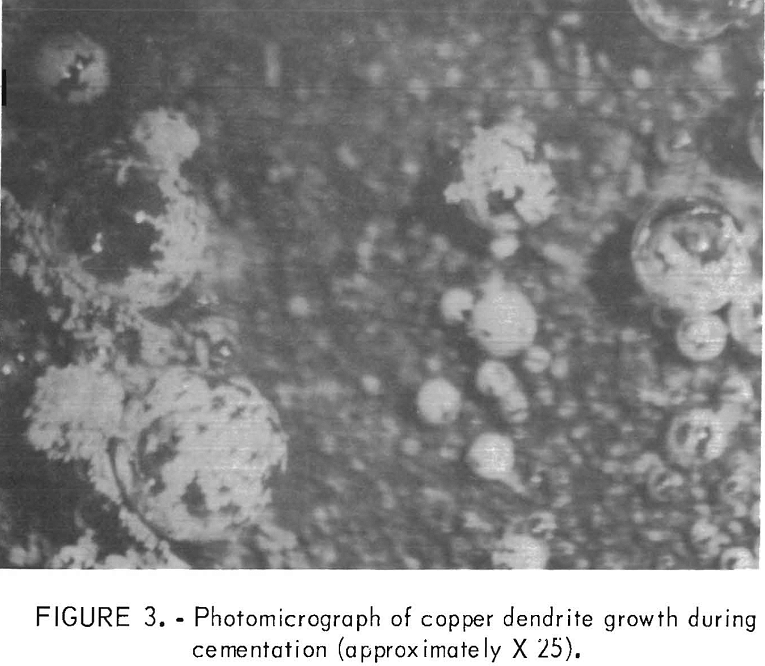
surface. The copper growth starts on the surface at the base of the bubble and continues until the bubble is encapsulated. The bubble apparently acts only as a surface for precipitation. The growth of copper dendrites on the surface indicates that copper precipitates on the existing copper deposit.
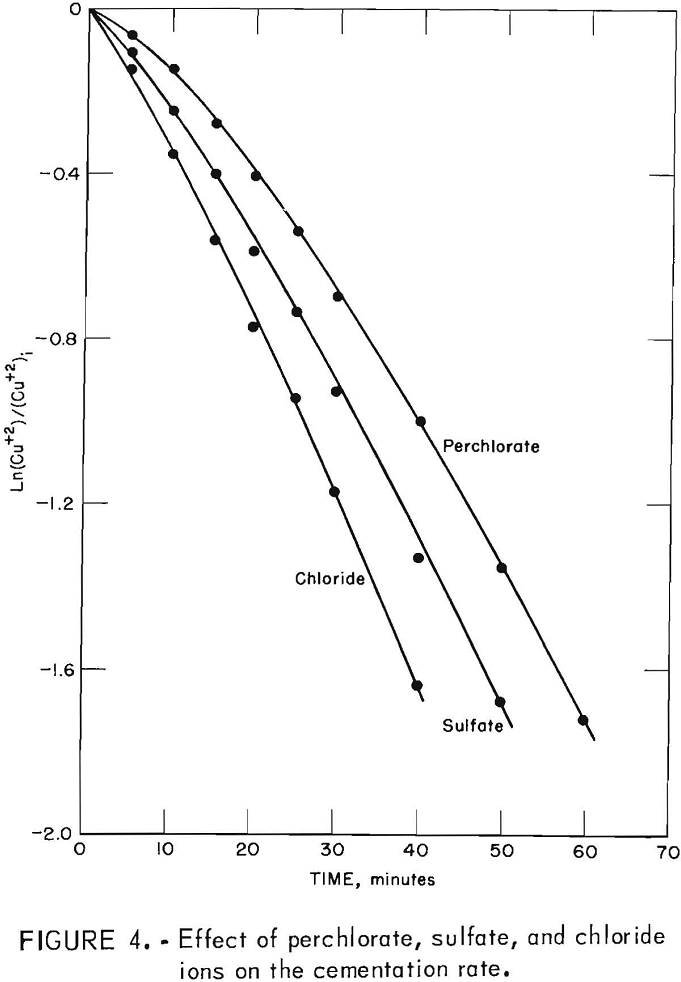
The next deposit effect appeared when the cementation reaction was studied as a function of anion type in the solution. Visual observation during tests using perchlorate, sulfate, and chloride ions showed clearly visible differences in the copper deposit. The perchlorate solution produced a smooth, coherent copper deposit which slowly broke away from the surface in thin sheets leaving a dull, granular deposit on the surface. The sulfate solution caused an initial smooth, coherent deposit which rapidly broke away from the surface leaving a dull, granular deposit. The chloride solution produced only a dull, granular deposit. The differences in the initial deposits were accompanied by changes in the reaction rate, as illustrated in figure 4, which is a first order kinetic plot. Figure 4 also shows that the rates for the three systems are not significantly different after the dull, granular deposit was established on the surface.
The most significant result of this study is that cement copper attached to the iron surface enhances the cementation rate. This effect was brought out most clearly when minus 20- plus 28-mesh quartz sand was added to the system as an abrasive and the reaction rate was significantly decreased, presumably due to the decrease in reaction area as copper was abraded from the surface. This experiment also shows that artificial removal of copper from the iron surface is detrimental to the rate of cementation.
Rate enhancement by the copper deposit also is shown clearly by the effect of initial copper concentration on the rate constant. Figure 5 illustrates the increase in the rate constant as the
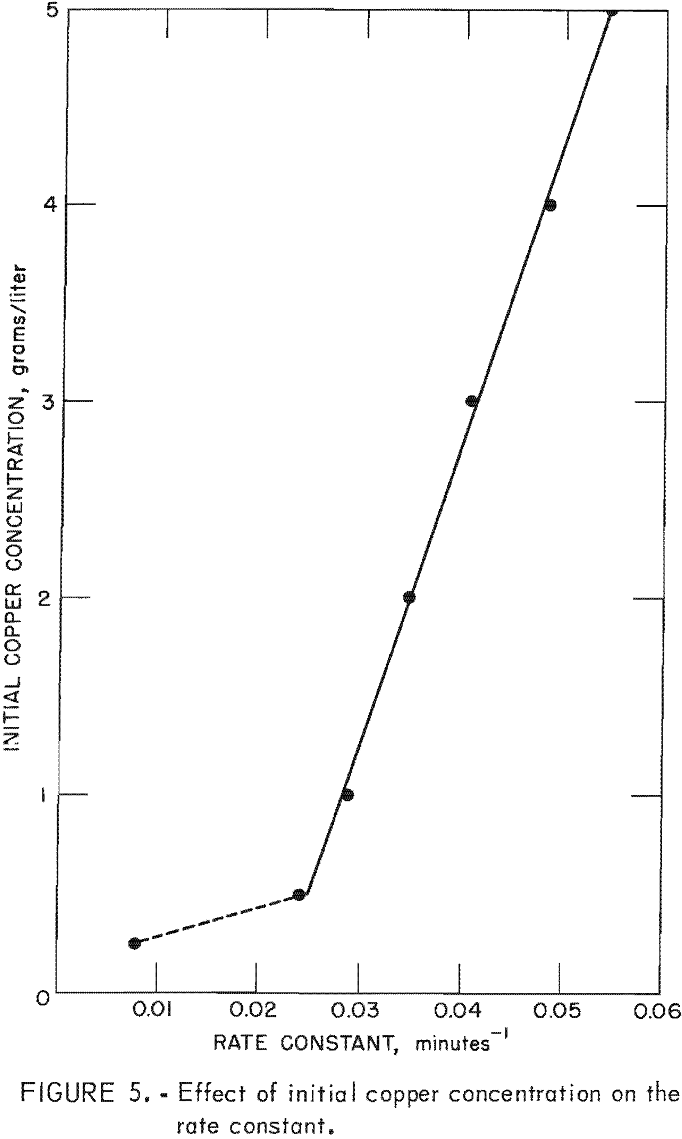
initial copper concentration is increased. Theoretically, the rate constant changes only as a function of temperature, and therefore, there must be an unknown variable affecting the reaction rate and causing an apparent change in the rate constant. The variation in the rate constant is attributed to differences in the copper deposit. Each initial copper concentration produces copper deposits having different specific surface areas for precipitation. The data in figure 5 appear to obey a regular relationship which will allow prediction of the enhancement to be expected for a given set of conditions. It may be possible to alter the operating procedure and design of cementation equipment to take advantage of the enhanced cementation rate resulting from deposited copper.
When additional cement copper was purposely added to the cementation system to form a slurry in which cementation occurred, the reaction rate was increased by a factor of as much as four. This result shows that discrete particles of copper can enhance the cementation rate when they are present in sufficient quantity. The conditions present in drum cementation most closely resemble a slurry of cement copper. However, similar conditions may exist to some extent in launders, cones, and columns.
The cement copper that is abraded from the iron during a normal cementation test contributes to rate enhancement by forming a slurry. The number of particles in the slurry, however, is statistically small, and significant rate enhancement is not apparent in these tests.
Conclusions
- During copper cementation, cupric ion precipitates on the copper deposit attached to the iron surface.
- The major anion present in a test solution causes a difference in the character of the initially deposited copper.
- The cementation rate is enhanced by the cement copper attached to the iron precipitant. This finding is contrary to the view expressed in current publications and patents.
- The cementation rate is also enhanced by the presence of discrete copper particles of a cement copper slurry.
