Table of Contents
The domestic base-metal-producing industry has been challenged with the task of reducing the amount of sulfur oxides and other pollutants emitted by smelters treating sulfide ores and concentrates. This challenge has been imposed by legislation aimed at reducing air pollution. Standards for both ambient air quality and allowable emissions have been proposed and already are in effect in some States.
Current Federal standards refer only to ambient air. There are two sets of standards. Primary standards, which protect the public healthy define how clean the ambient air must be so that it will not be harmful to human health. Secondary standards, which protect the public welfare, define how clean the air must be in order to protect against the known or anticipated effects of air pollution on property, materials, climate, economic values, and personal comfort. States are required to implement plans to achieve primary standards by 1975. No such time limitations have been legislated with regard to meeting the more stringent secondary standards.
The proposed and established State and local standards generally concern both ambient air quality and actual smelter emissions. Emission standards limit the amount of sulfur that can be discharged to the atmosphere, and are expressed as a percentage of the total sulfur charged to the smelter. Several Stales have established a 10-percent limit for the amount of sulfur that can be emitted as oxides from copper smelters. Montana has a 5- to 10-percent standard for lead and zinc smelters, depending on the feed rate to the smelter Several other States are considering similar emission standards.
Currently, there is a wide range of opinions concerning the minimum time in which an acceptable solution to the problem of sulfur oxide emissions can be achieved, while still maintaining our essential supplies and the strong competitive position of the United States as a primary producer of copper, lead, and zinc. The industry expects to be able to comply with the national primary ambient air quality standards within 3 years. The 10-percent sulfur emission limit may be more than is necessary to meet the ambient air standards. There may be serious and numerous difficulties; collection of gases from copper smelters is particularly troublesome. Of major concern is the fact that in order to comply with some State emission standards the companies must make capital investments to utilize process technology that has not yet been demonstrated on a commercial scale. Hence, within a few years, the industry may be faced with the problem of new plants using obsolete, outmoded, or costly non-competitive methods.
Production of sulfuric acid is the only proven technology for removing sulfur oxides from smelter gases. Most smelters are planning to install acid plants to capture as much of the sulfur dioxide as possible. However, acid production in the conventional copper smelter is practical only from converter gas. With tightly hooded converters, 50 to 70 percent of the sulfur oxides can be removed, at best. To achieve 90-percent removal of the sulfur, innovative or developing technology for smelting, handling, and concentrating the normally dilute gases generated in the reverberatory furnace will have to be incorporated. One process is a new version of the Asarco (American Smelting and Refining Co.) brimstone process which is being tested in a pilot plant at the company’s El Paso, Tex., smelter to produce elemental sulfur from gas containing 14 percent or more sulfur dioxide. Other possibilities include methods for separating and concentrating sulfur dioxide by scrubbing with absorbents such as ammonium sulfite-bisulfite or anhydrous dimethylanaline. Substituting flash smelters or electric furnaces for the reverberatory furnace is one approach to providing a strong total smelter off gas for conversion to acid. It is thought that enough sulfur will be removed in these plants to approach the 10-percent emission standard that has become law in Arizona, Nevada, Montana, and the Puget Sound area.
Some kind of neutralizing scrubbing system probably will be necessary to absorb the sulfur oxides from the weak gases from reverberatory and acid plant effluents at some smelters. Lime or limestone wet-scrubbing may be tried, since they are flexible methods and applicable to variable gas flows and sulfur oxide concentration. A tandem system, perhaps first scrubbing with limestone then with caustic soda, may be required. None of the alkaline scrubbing systems have been used on smelter gases, and difficulties have been encountered in pilot-plant operations on gases from coal-fired furnaces. It is doubtful that smoothly operating scrubbing units will be on-stream in less than 5 years.
The expanded production of acid which is expected to accompany the sulfur dioxide, control efforts is causing considerable concern. In many cases, smelters are not near major markets for sulfuric acid, and it is likely that unmarketable acid will have to be neutralized and disposed of by impounding.
Collectively, the copper smelting industry estimates that capital expenditures of about $600 million will be required to make the changes necessary to achieve compliance with a 10-percent sulfur emission standard. This investment, combined with the attendant operating costs, will result in an average increase in smelting costs of about 4 cents per pound of blister copper over the current cost, which is estimated to range from 4 to 6 cents per pound. The individual company estimates were examined by Bureau of Mines engineers and were judged to be reasonably accurate.
A study made of the copper industry by an independent engineering firm estimated that the capital investment required to implement controls at 12 western smelters that would allow compliance with Federal ambient air quality standards would be approximately $600 million. This includes the capital cost of new plants necessary to maintain industry wide levels of production, assuming some operations are curtailed to meet the most stringent and universal emission standards.
The Environmental Protection Agency has estimated that capital cost would range from $250 million to $270 million. The difference in the cost estimates is a matter of the interpretation; the industry and the Environmental Protection Agency differ with respect to how much of the capital expenditures should be appropriately charged to pollution control and how much to facility improvements or new copper production capacity.
A detailed, industry wide study of control costs for limiting sulfur oxide emissions from lead and zinc smelters has not been made. However, a partial survey made by the Bureau of Mines indicates at least a $100 million capital investment will be required. Smelting cost, currently estimated to be 2 cents per pound of lead and 6 cents per pound of zinc, probably will increase 2 to 4 cents for lead, and about 1½ cents for zinc.
The industry may find it difficult to pass the additional costs on to the ultimate consumer because of the worldwide pricing structure of copper, lead, and zinc. If the costs must be absorbed by the companies, corporate profits could be so seriously reduced that the continued operation of some smelters may not be economic.
There is reasonable assurance that, given the necessary time for development and demonstration, an engineering capability will emerge that can cope with smelter gases in an efficient and effective manner. In the meantime, concerted effort should be made to develop improved technology and to formulate time schedules that reflect a reasonable balance between what must be done eventually and what can be done now. This position is fortified by the magnitude of the capital investment that will be required to make necessary smelter modifications and to incorporate gas treatment facilities.
Introduction
Sulfur oxides have been designated as one of the more severe air pollutants, and consequently, air pollution standards for sulfur dioxide have been promulgated which encompass the domestic copper, lead, and zinc smelters which treat sulfide ores and concentrates. The evolution of sulfur dioxide at these base metal smelters is inherent to conventional smelting practice. Moreover, a reduction in the amount of sulfur dioxide emitted poses many difficult technological problems which are taxing the ingenuity of industry for a satisfactory solution.
The implications of the problem are shown by the fact that supply-demand forecasts for copper, lead, and zinc in the United States consistently show demand exceeding domestic production. The United States leads the world in copper production and is a major producer of lead and zinc. Collectively, the value of smelter output for the three metals amounts to $2.3 billion yearly. The extent to which domestic sources can meet future requirements is likely to decrease. As copper, lead, and zinc are key commodities in the industrial economy, increased production should be encouraged, although it is recognized that imports will have to supply an increased percentage of future U.S. demand. Cost reductions at every stage from exploration through fabrication will have to accommodate not only the continuously decreasing tenor of raw materials but also be effective enough to assure a viable competitive position with respect to foreign operations.
This study provides an evaluation of the possible impact of sulfur dioxide control on sulfide smelting, including a review of the existing or proposed regulations for controlling smelter pollution, the magnitude of the problem as it affects the domestic smelting industry, the state of the art of sulfur dioxide emission-control technology, and the efforts that are being made by industry to develop acceptable technology for abating or controlling smelter pollution.
Significance of the Industry
Salient statistics on copper, lead, and zinc primary smelting operations are shown in table 1. In summary, copper, lead, and zinc smelters produced 2.4 million tons of metal in 1960 and 3.2 million tons in 1970. It is anticipated that growth will continue for copper and lead smelter production and that by 1980, total smelter output will increase to more than 3.6 million tons During the decade 1960-70, the value of nonferrous smelter output increased from $1.1 billion to $2.3 billion annually.
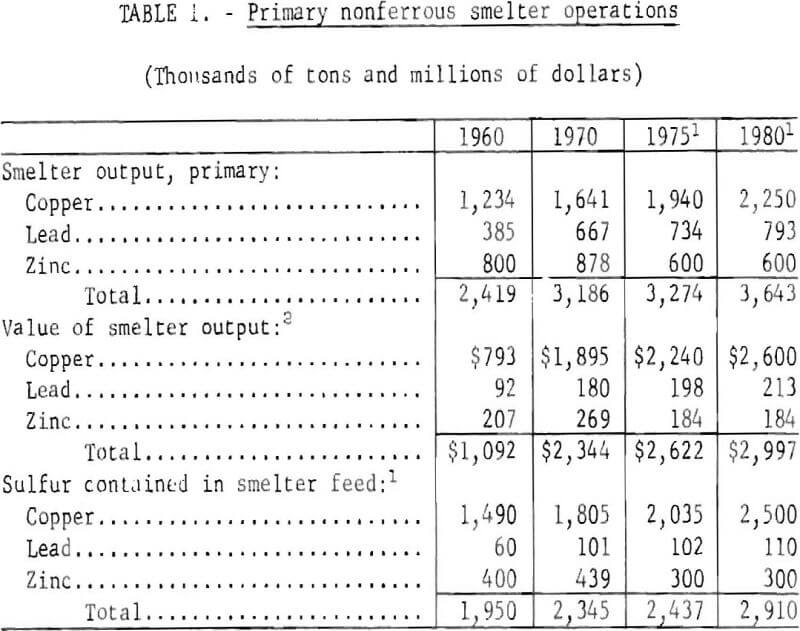
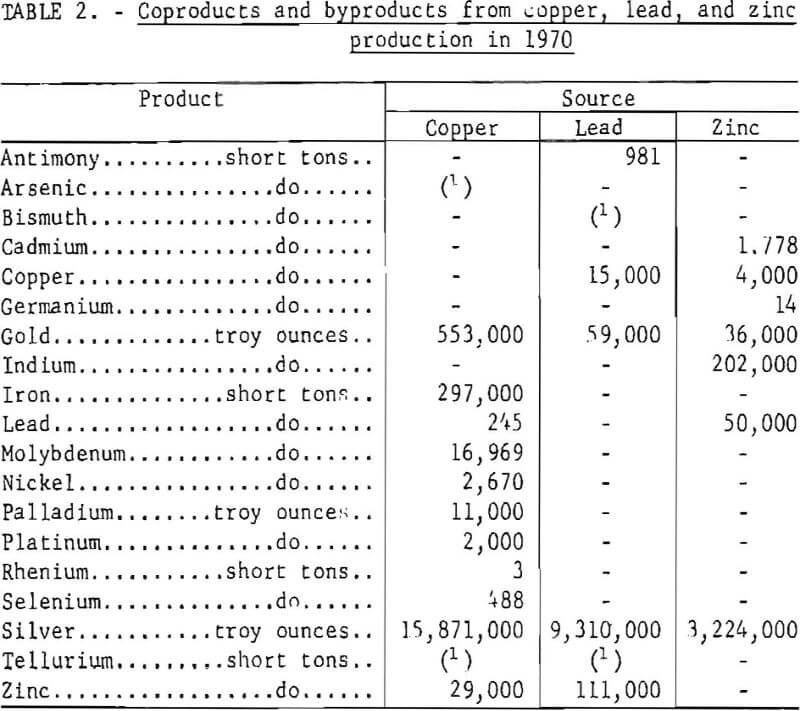
The supply of a host of byproducts and coproducts as shown in table 2 depends upon the rate of copper, lead, and zinc production and the technology of processing and refining. In one instance, production of copper from the porphyry deposits in the Western United States provides the only domestic source of rhenium, a metal of increasing importance as a catalyst for reforming gasoline. Collectively, the 1970 value of byproducts and coproducts recovered from domestic base metals operations amounted to more than $250 million. Any reduction in smelter production would have a like effect on supply of these elements.
The growth of the base metals industries has been accompanied by numerous problems. One of the most pressing of these problems is disposal of sulfur, an integral part of the ore, without harmful effects to the environment. The quantities of sulfur contained in the smelter feeds are indicative of the magnitude of the problem. In 1960, smelter feeds contained less than 2 million tons of sulfur; by 1970, this had increased to 2.3 million tons. It is estimated that by 1980. 2.9 million tons of sulfur will enter nonferrous smelters annually in copper, lead, and zinc concentrates. Of the sulfur entering the smelters in 1960, 387,000 tons or 20 percent was recovered as sulfuric acid; in 1970, 600,000 tons or 26 percent of feed sulfur was recovered as acid.
Copper
Significant production of copper began in the United States about 1845. Until World War II, production exceeded consumption and major quantities were exported. During the period from 1915 to 1939 the United States was the largest exporter of refined copper in the world. About 1940, the United States shifted from a net exporting nation to a net importing nation. During the period, 1966-70, 17 percent of domestic demand has been supplied by net imports, principally from Chile, Peru, and Canada.
Because U.S. production is inadequate for all needs, copper is designated as one of the strategic materials to be stockpiled for U.S. protection in case of war. Return of the United States to a position of self-sufficiency is not likely under foreseeable conditions, and further deterioration of the domestic copper mining industry could be disastrous in times of emergency. The industry has been confronted with economic problems relating to all phases of activity resource development, mining extraction, marketing, and pollution abatement.
The United States has been the leading copper-producing country in the world since 1883. Arizona was the leading copper-producing State in 1970 with 54 percent of total domestic output, followed in order of production by Utah, New Mexico, Montana, Nevada, and Michigan. These six States accounted for 97 percent of the 1.7 million tons of 1970 mine production. Open pit mines supplied 84 percent of mine output and underground mines 16 percent.
Virtually all copper ore is treated at concentrators near the mines. Concentrates are processed at 16 smelters-eight in Arizona and one each in Utah, Michigan, Montana, Nevada, New Mexico, Tennessee, Texas, and Washington. In 1970, copper smelting capacity in the United States totaled 9.2 million tons of charge, estimated to represent 1.8 million tons of smelter product. Four companies accounted for nearly 80 percent of the smelter capacity.
Refinery capacity is estimated to be 2.7 million tons, of which 88 percent is electrolytic refining capacity and 12 percent is fire-refining. About 60 percent of the electrolytic refinery capacity is accounted for by six plants on the Atlantic coast in New York, New Jersey, and Maryland. Arizona, Missouri, Montana, Texas, Utah, and Washington each have one electrolytic refinery, which in total account for the remaining 40 percent. Fire-refined copper is produced in Michigan, New York, New Jersey, New Mexico, and Texas.
In 1970, consumption of refined copper was 2.0 million tons. An additional 0.7 million tons was consumed as scrap in production of alloys, chemicals, and other products. The refined consumption was 61 percent by wire mills, 37 percent by brass mills, and 2 percent by others. Scrap copper was consumed by smelters to produce refined copper and copper alloy ingots, and also by brass mills.
The largest use of copper is in electrical equipment and supplies, which accounts for about one-half of demand. The manufacture of electric motors, power generators, motor-generator sets, electrical controls, and related apparatus requires the use of copper for the best performance. Copper is vital to industrial and military needs in many other use areas such as in construction, transportation, industrial machinery, and ordnance.
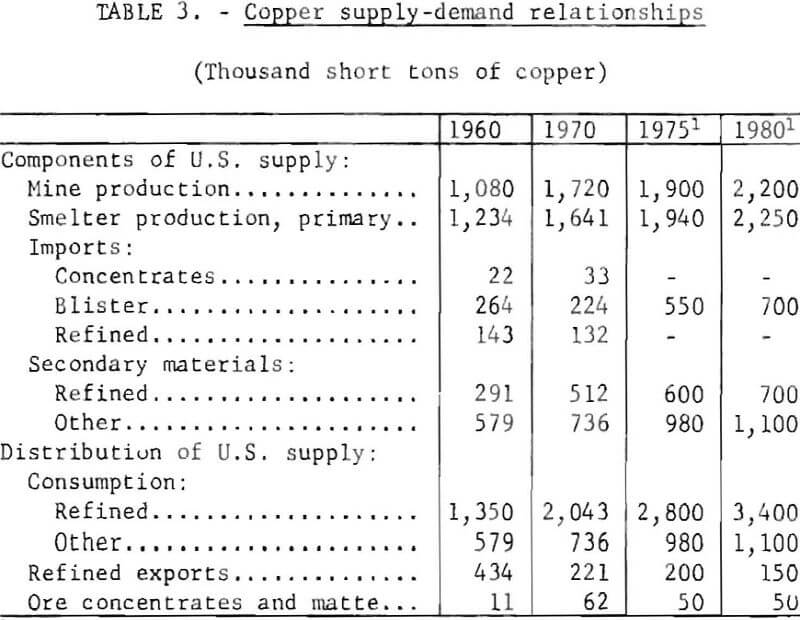
Table 3 shows the salient components of domestic supply and demand for copper for 1960 and 1970. Also shown are projections for 1975 and 1980. These projections assume that the Federal Ambient Air Quality Standards will be implemented without significant disruptive effect on normal domestic supply patterns.
The major copper-producing countries of the free world, other than the United States and Canada, are emerging nations which have a critical need for employment that the copper smelting and refining industry provides. Also, these countries are in need of hard currency exchange credit which they obtain by the export of copper.
Prior to 1970, U.S. smelting capacity was greater than required for treating domestic materials and part of the excess capacity was used to treat substantial quantities of foreign crude materials. This condition was reversed in 1970. In mid-1970, a major copper smelting company, which provides custom smelting service, notified all customers that new pollution restrictions made it necessary to invoke the force majeure clause in their contracts to the extent of reducing the amount of ore and concentrates accepted by 15 percent. Thus, the demand by domestic producers for smelter capacity exceeded availability, and arrangements were made to treat the concentrates in foreign smelters. The copper content of the concentrates exported for treatment increased from 979 tons in 1969 to 58,450 tons in 1970. Although the increase in exports cannot be attributed solely to pollution regulations, it reflects a reduction in effective capacity at domestic smelters when demand is high.
Lead
In 1970, U.S. mines and smelters accounted for 572,000 and 666,730 tons of lead, respectively, which represents 15.3 percent and 18.3 percent of the world’s output, indicating the strong position of this country as a lead producer. Some 120 domestic mines reported lead ore production in 1969, many of which produced coproducts of copper, zinc, silver, and other elements. Missouri is the leading lead ore-producing State, followed by Idaho, Utah, Colorado, and other States. The domestic lead-mining industry is backed by ore reserves estimated to contain 36 million tons of recoverable lead.
Domestically produced lead ores and concentrates, often comingled with similar raw materials imported notably from Canada, Mexico, Peru, and Australia, are treated in seven smelters; three in Missouri and one each in Idaho, Montana, Texas, and Utah. An eighth smelter, in California, was recently closed. Four of the operating smelters have access to ancillary facilities for refining. Two separate lead refineries treating domestic and imported base bullion are operated in Nebraska and Illinois.
In 1970, the industry consumed 1.36 million tons of new and reclaimed lead. Approximately 42 percent of this went to manufacture of storage batteries, 28 percent to various metal products, 20 percent to gasoline additives and other chemicals, and 7 percent to pigments.
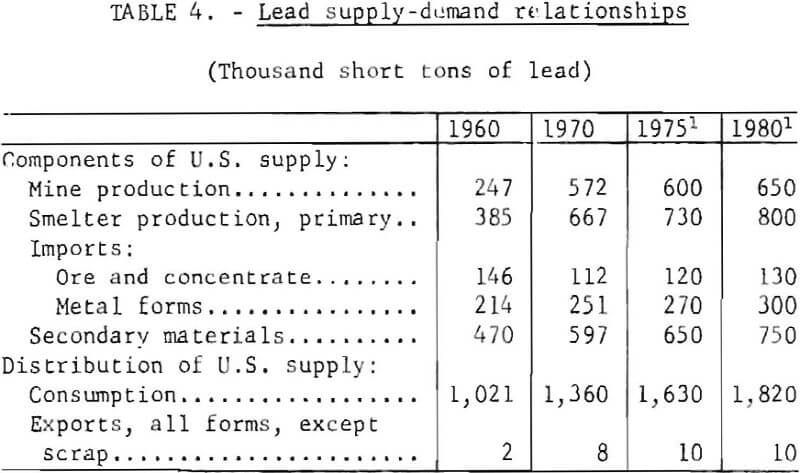
Salient components of the domestic supply and demand for lead in 1960 and 1970, together with projections for 1975 and 1980, are given in table 4.
Zinc
Of the total 1970 U.S. supply of zinc, 31.6 percent was from domestic mine production and 31.1 percent was from imported inc concentrates. The mine production and imported concentrates supplied the raw material feed for four electrolytic refineries and eight smelters. Since 1953, zinc smelters and refineries have been dependent on foreign concentrates for about half of their supply.
Competition has increased for these foreign concentrates because of the recent erection of zinc plants in other countries. As a result, during 1970, companies paid higher prices for concentrates while operating under pressure for a lower zinc price because of slackening demand. During the first half of 1971, four plants were closed, including three retort operations and one electrolytic refinery, reducing domestic annual capacity from 1,251,000 to 993,500 tons.
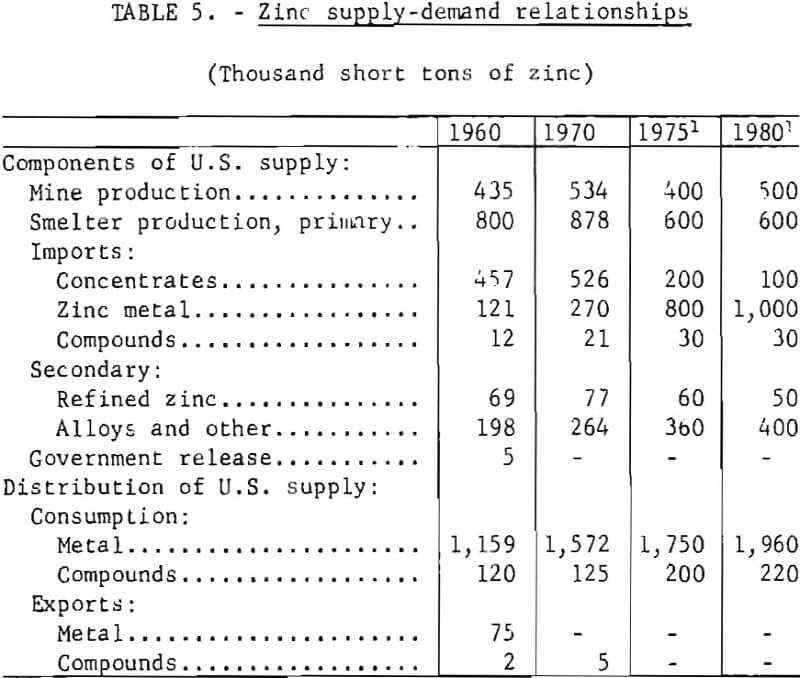
Influence of the zinc industry extends to other industrial activities which depend upon zinc for their products, including die-casting plants, galvanizing operations, and brass mills. Salient components of the domestic supply and demand for zinc in 1960 and 1970, together with projections for 1975 and 1980, are given in table 5. The estimated projections assume a decline in annual zinc production capacity to 700,000 tons, resulting from additional closures of smelting facilities and lack of foreign concentrates for refinery feed.
Dimension of the Problem
Air Pollution Regulations
Air pollution problems created by smelting sulfide ores and concentrates are not new, but date back to the very inception of smelting. Nor have they been ignored by either the industry or the public. The history of smelting is replete with examples of legal actions instituted by individuals against smelter operators, in which claims for damage to property were the basis of action. Generally, these were settled by purchase of affected land or by cash payments to the claimants. Prevention was sought by building high stacks that dispersed the fumes in a manner that obviated any gross damage to far-lying property.
The growth in industrial activity throughout the Nation and the proliferation of automobiles has increased air pollution throughout the United States, though primarily in the cities. This has led to a general demand for some sort of national control over air pollution. Emissions caused by smelters are only a small part of the national situation, but; the nonferrous smelting industry was inevitably drawn into the larger problem because the smoke and sulfur- bearing gases contribute to the total air pollution burden and are the major contributors in certain regions of the country.
The demand for some form of national control over air pollution subsequently led to the enactment of a series of Federal statutes dating back to a basic law of 1963. These have since been amended and revised several times.
The latest revision, the Clean Air Act of 1970, established the principle that the Federal Government promulgate ambient air quality standards and the States bear the responsibility for the development of plans for implementation. In all cases, however, State regulations must at a minimum provide for the attainment and maintenance of the Federal ambient air quality standards.
Prior to the effective date of Reorganization Plan No. 3 of December 2, 1970, the authority for Federal investigation and regulation was invested in the hands of the National Air Pollution Control Administration (NAPCA) of the Department of Health, Education, and Welfare. Because the problem of air pollution from nonferrous smelters posed a problem that was unique, NAPCA negotiated a contract for technical services with Arthur G. McKee and Company in December 1967, to prepare a comprehensive evaluation of the problems existing in this industry as related to air pollution. A report on this subject was published in June 1969.
Late in 1970, a general reorganization of Federal pollution-related functions placed the national problem of air pollution under the direct authority of the Environmental Protection Agency (EPA). Section 109 of the Clean Air Act, as amended December 31, 1970 (Public Law 91-640), directed the Administrator of EPA to establish national primary and secondary ambient air quality standards. Proposed standards were published in the Federal Register, v. 36, No. 21, January 30, 1971. The final standards were issued April 30, 1971, and constitute the criteria on which future State and local regulations must be based. Each State is required to adopt regulations and submit implementation plans to EPA by January 30, 1972. Within 4 months of that date, or by May 30, 1972, EPA will either approve or disapprove each State plan. The schedule for submission of plans to achieve primary air quality standards is fixed, but may be extended an additional 18 months for secondary standards. The plans must provide for compliance to a primary standard as expeditiously as possible, and in no instance later than 3 years from the day of approval, or May 30, 1975, and for compliance to a secondary standard within a reasonable time. In the case of a primary standard, EPA may grant a 2-year extension beyond May 30, 1975, if technology is not available.
In the meantime, the various State and local agencies concerned with air pollution have been engaged in proposing and establishing regulations of their own. As might bu expected, they have differed widely, both as to scope and degree of regulation. Some are comprehensive, others are meager in detail.
Current Federal standards refer only to ambient air. There are two sets of standards. Primary standards, which protect the public health, define how clean the ambient air must be to protect human health. Secondary standards, which protect the public welfare, define how clean the air must be in order to protect against the known or anticipated effects of air pollution on property, materials, climate, economic values, and personal comfort.
The proposed and established State and local standards generally concern both ambient air quality and actual smelter emissions. Ambient air standards refer to pollution in the air at the property boundary of the smelter operation generally at ground level. As with the Federal standards, they are usually designated as maximum sulfur dioxide concentrations for various time intervals Emission standards limit the amount of sulfur that can be discharged to the atmosphere, and are expressed as a percentage of the total sulfur charged to the smelter. Several States have established a 10-percent limit for copper smelters. Montana has a 5- to 10-percent limit for lead and zinc smelters, depending on the feed rate to the smelter. Several other States are considering similar emission standards.
Ambient air quality standards generally provide for fluctuation in the smelter operation. They have allowed for short periods of time in which ambient air limits for sulfur dioxide may be high, provided that the average over a longer period of time is within prescribed limits of tolerance. Conversely emission standards based on sulfur burden to the smelter are more restrictive in that they limit the alternatives for achieving an acceptable sulfur oxide concentration in the ambient air. Further, they may fail to consider how the unique or unusual characteristics of each smelter relate to local or regional pollution.
To some extent, State and local regulatory agencies have recognized that time must be allowed for the industry to fully comply with established standards because of the technological problems that must be solved, and the lead- time required to construct the necessary air pollution control facilities. For the most part, this has been set at about 3 years. However, the time allowance is contingent upon serious, continued action on the part of the smelter operators to change their operation to meet set standards.
The Federal ambient air quality standards and the proposed and established State standards applying to sulfur emissions at copper, lead, and zinc smelters are summarized in the appendix.
Smelting Practice
Copper
Copper, as it occurs in ore, can be classed as three main types-native, oxide, and sulfide. Most of the world copper production comes from low-grade sulfide ores from which the sulfide copper minerals are readily concentrated by flotation. The finely ground flotation concentrates consist of copper sulfides, iron sulfide, and residual gangue. The purpose of smelting is to separate the copper from the iron, sulfur, and gangue. The thermodynamic relationships between these principal ingredients form the basis for the three major steps: roasting to remove excess sulfur, smelting in a reverberatory furnace to remove gangue and to form a copper-iron-sulfur mixture called matte and oxidation of the matte in a converter to form a separable iron slag and to burn the sulfur away from the copper. Each of these steps generates sulfur oxides.
Two types of equipment are used for roasting-multiple-hearth and fluosolids roasters. Operation of the fluosolids roaster generally is autogenous in that no fuel is required other than that needed for preheating of the roaster prior to start-up. Operation of multiple-hearth roasters, on the other hand, may or may not be autogenous, depending primarily on the ratio of copper to sulfur in the material roasted. Owing to the wide variation in composition of concentrates and other copper-bearing materials treated at primary copper smelters in the United States, the sulfur dioxide content of roaster gases may range from as low as 1 percent when it is necessary to burn auxiliary fuel to as high as 12 percent in autogenous fluosolids roasting.
The capacity of a multiple-hearth roaster ranges from 150 tons of feed per day to as high as 800 tons per day. The fluosolids roaster, a fairly recent development, can have a higher capacity; a single roaster may handle as much as 1,200 to 1,500 tons of feed per day.
When sulfur content of the smelter feed is high, more sulfur than copper, roasting to reduce sulfur content before smelting is attractive and may reduce the amount of sulfur discharged from the reverberatory furnace to less than 10 percent of the total feed sulfur. When less sulfur than copper is in the feed, roasting is not practiced, and the sulfur discharged in the reverberatory off gas may be as high as 30 percent of the total sulfur.
Calcined concentrates, if roasting is practiced, or unroasted concentrates (green feed) are smelted in a reverberatory furnace fired with oil, gas, or pulverized coal. The large volume of gas generated by the burners produces an off gas containing only a dilute concentration of sulfur oxides. The sulfur dioxide content of the furnace gas generally ranges from 0.25 to 1 percent when smelting calcines or low-sulfur feed. When concentrates rich in sulfur are fed directly to the furnace, the sulfur dioxide content of the gas may be as high as 2.5 percent. The strength of the gas also depends upon the fuel used and the operating conditions of the furnace.
The process of removing iron and sulfur from copper in a converter is a controllable operation, but unlike the roasting and smelting steps, it is a batch process. The sulfur dioxide content of the off gas may range from 3 to 12 percent during the converting cycle.
When multiple converters are in use, the cycles can be staged to hold the strength of the combined off gases within a range of 4 to 6 percent, which is suitable for sulfuric acid manufacture.
Lead
Domestic primary lead is derived from the sulfide mineral galena. This mineral contains slightly less than 14 percent sulfur by weight, and the overall sulfur dioxide problem is not as acute as for the copper smelter.
Lead smelting is the same at the seven primary lead smelters in the United States; sintering of concentrates, reduction of the sinter in a blast furnace to obtain bullion, and refining. In the smelting process only the sintering step evolves enough sulfur dioxide to create a serious air pollution control problem. Sintering converts the sulfide concentrates to the oxide form, and simultaneously produces a strong, porous feed for the blast furnace.
Older sintering machines are of the downdraft type, where the air is drawn through the charge by windboxes located below the continuous conveyors made of grate-bar pallets. The combustion of the sulfides provides enough heat to sustain the reaction with the temperature controlled at about 1,400° F Essentially unrestricted infiltration of air generally dilutes the sulfur dioxide gas from the machines to about 1 percent.
Newer smelters use updraft sintering machines which have the advantage of producing a 4- to 6-percent sulfur dioxide gas which can be fed into a sulfuric acid plant. Sintering removes about 85 percent of the sulfur from the charge. Only about 1 percent of the remainder is discharged as sulfur dioxide from the subsequent furnacing operations; the balance reports to the slag. The off gas is cooled and the dust load is recovered by bag filters or by electrostatic precipitators. Three smelters use the clean gas for sulfuric acid production.
The sulfuric acid produced at lead smelters is generally dark in color because of organic impurities introduced into the gas stream. These impurities result from burning of the residual flotation reagents on the concentrates during the sintering step. Because the acid is colored, its market value is generally less than that of the clear acid produced from sulfur or copper smelter gas.
Calcine from the sintering machine is reduced with coke in a vertical blast furnace. The furnace product is lead bullion, 95 to 99 percent lead. It is refined primarily by a series of drossing steps in large kettles. Slag from the blast furnace is treated to recover lead and zinc or it is recycled to the sintering machine after granulating. Blast furnace off gas is cooled by air dilution and then filtered to recover particulate matter.
Zinc
Most zinc and zinc oxide is produced from sulfide ores, although occasion ally some oxide ore is available for processing along with the sulfide materials. In the smelting process the notation concentrates are first roasted to convert the sulfides to oxides. The roasted product is frequently sintered or calcined to obtain a desired physical condition. The zinc is extracted by selective vaporization in retorts and then condensing, or by dissolving the oxide in sulfuric acid for electrowinning.
A typical concentrate contains approximately 60 percent zinc, 30 percent sulfur, and 5 to 10 percent iron. In roasting zinc sulfide concentrates, 90 percent of the sulfur is eliminated. Some of the zinc oxide reacts with sulfur gases to produce zinc sulfate, the quantity being controlled by temperature and other equilibrium conditions. When the roasted ore is used for distillation methods, sulfur in any form will tie up approximately twice its weight in zinc in the residues, therefore roasting is carried on to eliminate as much sulfur as possible (dead roast). When used in the electrolytic method, it is desirable to have the calcine contain 2 or 3 percent of the sulfur as sulfate to replace the sulfate ion lost with the sulfuric acid in the leach residues.
Roasting is accomplished in several different types of equipment ranging in capacity from 40 to 350 tons of concentrates per day. The newer type suspension or flash, fluidized bed, or fluidized column have larger capacities and yield treated off gases rich enough in sulfur dioxide for use as feed to an acid plant. The sulfur dioxide content of the off gas from these units ranges from 10 to 13 percent, but generally this is decreased to 6 to 8 per-cent in subsequent cooling and cleaning operations. Ropp roasters and multiple-hearth roasters are among the older types still in service. The off gases from these types of roasters contain only about 1 percent or less of sulfur dioxide. These gases are too dilute for efficient conversion to acid in a contact plant, and therefore are discharged to the atmosphere.
The subsequent steps in zinc extraction generally represent insignificant sources of sulfur dioxide, and in none of them is sulfur recovery practiced. The sulfur content of calcine from roasting ordinarily does not exceed 3 per-cent and may be as low as 0.1 percent. Only when sintering is conducted with raw concentrates as a part of the feed does some step other than roasting produce significant quantities of sulfur dioxide. The waste gases from sintering contain 0.1 to 2.4 percent sulfur dioxide, while in calcining the sulfur dioxide range is 0.1 to 2 percent.
Reduction of the calcine or sinter to metal by retorting produces very little sulfur dioxide and production of metal by leaching and electrolysis produces essentially none.
Of 12 operating plants, including four roasting facilities, eight have sulfuric acid plants operating on roaster gases. Some of these plants are currently meeting ambient air quality standards, and a few will meet the emission standard which allows no more than 10 percent of the feed sulfur to be discharged into the atmosphere.
Horizontal retorts for distilling zinc are faced with a difficult air pollution problem, in that the effluent from the condensers of the retorts contains minute particles of condensed zinc oxide. These particles appear as a white smoke. Although little sulfur oxide is contained in this gas, stringent requirements for reducing particulate emissions could be prohibitive for horizontal retorting. Systems for collecting the many small gas streams for cleaning, or for cleaning each individual stream have not yet been devised. One company has reported a test being conducted to determine if particle collection is practical, but because of the complexity of the problem, it is doubtful that satisfactory collection technology will be developed in the near future.
Smelter Gas
Handling the gases discharged from smelter furnaces presents major engineering problems. This is particularly the case in copper smelting. Large volumes of hot gas containing rock dust and other particulate material must be cooled, cleaned, and channeled, either to an acid plant or to the stack for venting.
The gases normally contain a small amount of sulfur trioxide. Most processes proposed for removing sulfur dioxide require a preliminary step where the sulfur trioxide is removed from the gas stream. In the past, the smelter stack was considered adequate for dispersing the smoke. Inasmuch as unrestricted discharge of smelter gases will no longer be permitted, the smelter operators must now implement processes to substantially reduce the sulfur oxides discharged into the atmosphere. Extensive gas handling and cleaning systems will have to be installed and acid plants will have to be erected to capture the gases and convert them to sulfuric acid for use or disposal. Figures 1, 2, and 3 show aerial views of copper, lead, and zinc smelters, respectively, two of which have facilities for acid production.
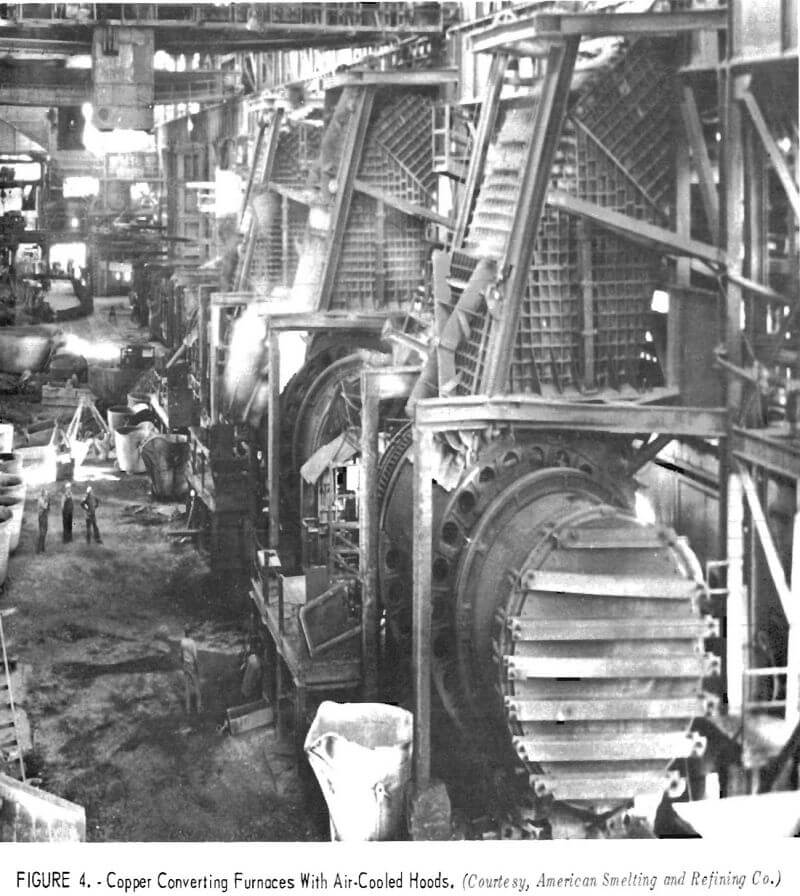
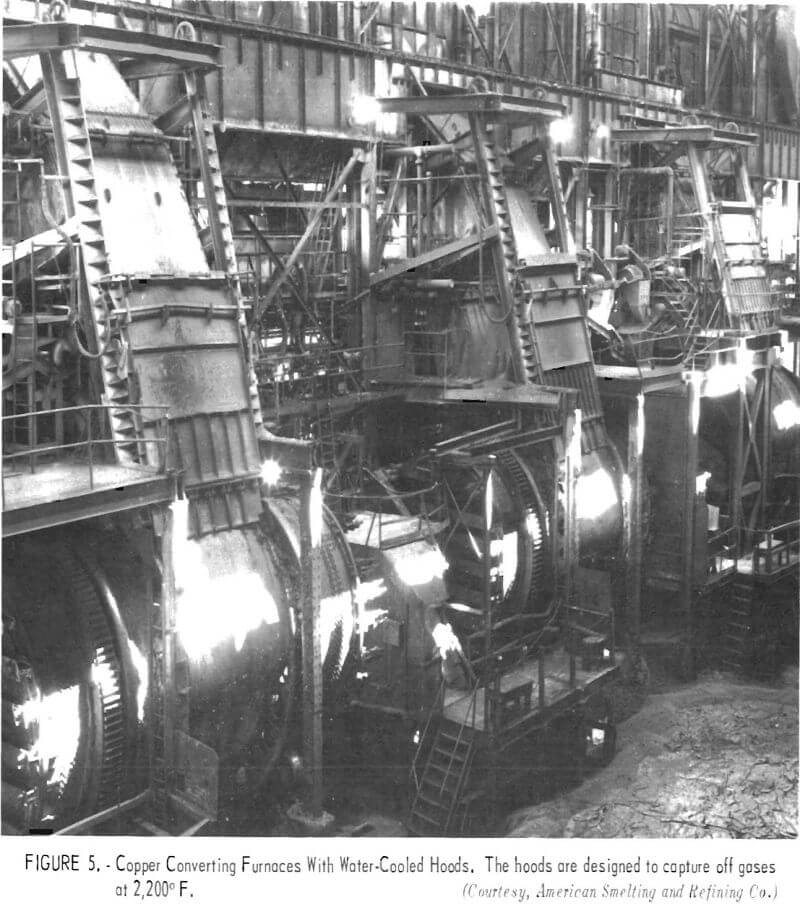
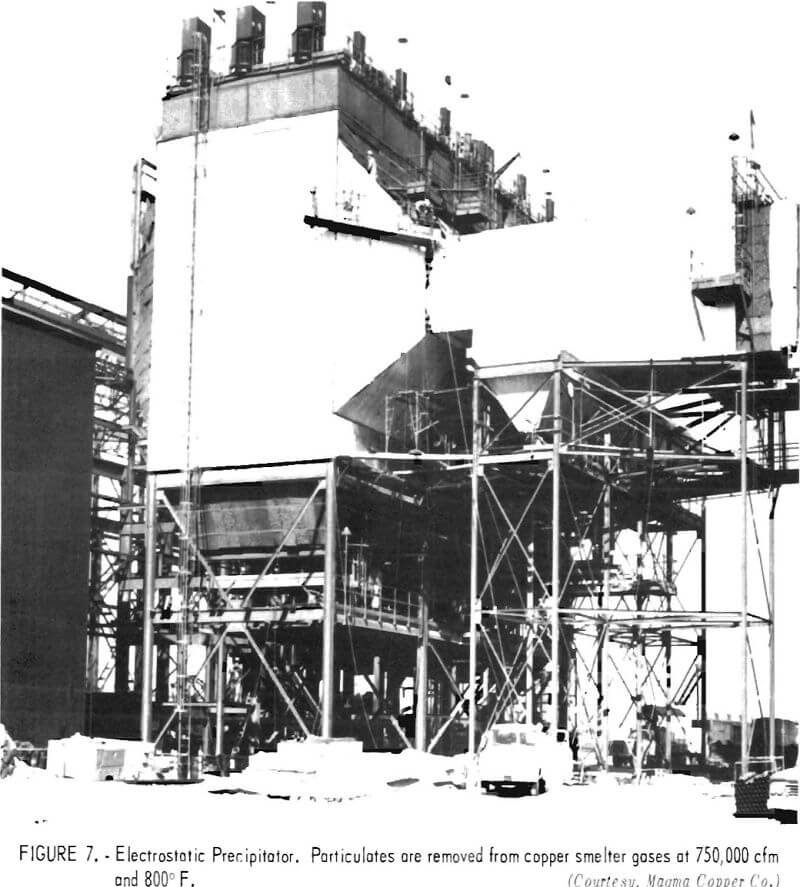
To prevent dilution of furnace gases, the hoods, flues, and other duct-work must be as airtight as practical. For the copper converter furnace, this is a particularly expensive installation because of the high temperature of the exit gas and the nature of the converting operation. Figure 4 shows converters equipped with waffle-pattern, air-cooled hoods. During operation, the temperature of the gas emerging from the furnace is lowered by permitting air to enter the system between the furnace and the hood. The cooling air prevents damage to the hood and the supporting superstructure, but also dilutes the concentration of sulfur dioxide in the collected gas. Figure 5 shows similar converters with water-cooled hoods designed to capture the gases for acid manufacture. With these hoods, a relatively tight seal can be made between the converter and the hood because it is not necessary to mix the furnace gases with cooling air. Once collected, hot converter gas is generally passed through large balloon flues where the heavy particles settle out and are recovered for return to the reverberatory furnace. Figure 6 shows a balloon flue being constructed at a copper smelter to handle 500,000 cubic feet of gas per minute. The lighter particles in the gas are recovered by electrostatic precipitators; a recently installed precipitator is shown in figure 7.
At lead smelters the hot gases from the sintering operation and the blast furnace are channeled through a baghouse to recover the particulates. A typical baghouse is shown in figure 8.
After collecting and cleaning, the gases are fed to a sulfuric acid plant, shown in figure 9. Figure 10 is a view of a total acid plant complex recently constructed at a western copper smelter.







Current Practice for Sulfur Dioxide Control
Currently, there are three methods in use for reducing the sulfur dioxide concentration in the atmosphere surrounding a smelter. They are: (1) the tall stack; (2) the tall stack coupled with a contact sulfuric acid plant; and (3) closed-loop control.
Of the three methods, the tall stack is the simplest. It discharges the sulfur dioxide at such heights that the gas is diluted when dispersed into the lower atmosphere. The trend has been toward taller and taller stacks. For example, the average height of stacks for coal-burning power plants in the United States has been reported to have increased from slightly less than 250 feet in 1960 to more than 600 feet in 1969. Even now, the International Nickel Company of Canada is constructing a stack 1,250 feet tall at Copper Cliff, Ontario, to meet the air quality standard of the Ontario Air Management Branch. Even if other processes for removing sulfur dioxide from the smelter gas are adopted, the tall stack will still be an important control method. It is not likely that any process will emerge in the near future that will allow discharge of the tail gas at or near ground level.
The second method of reducing the sulfur dioxide concentration in the atmosphere couples the tall stack with a contact sulfuric acid plant. Production of sulfuric acid by the contact process is well established and is the only process now being used at smelters to remove sulfur dioxide from stack gases. Acid plants require at least 3½ to 4 percent sulfur dioxide in the feed gas and cannot economically treat weaker gases. The requirement of strong gas for acid production is one reason why many processes have been investigated for concentrating the sulfur dioxide from dilute gas streams. With current copper smelting practice, production of acid from the roaster and converter gases could reduce the sulfur oxides emitted to the atmosphere by 55 to 70 percent. Recovery at lead and zinc smelters will vary, depending on the type of sintering and roasting equipment used.
The third method of controlling sulfur dioxide pollution is production curtailment when adverse weather conditions prevail. This “closed-loop control” employs a number of sulfur dioxide monitoring devices which are strategically located at different distances in the area surrounding the smelter. These monitoring devices sense the rise or fall of sulfur dioxide concentration in the atmosphere and report to a control center where the information is analyzed with respect to short-term weather forecasts. Based on this information, the smelter operation is adjusted to reduce the discharge of sulfur dioxide below the allowable level of contamination.
State standards prohibiting sulfur emissions greater than 10 percent of that contained in the smelter feed may at this time be met by only a few plants. If less restrictive emission standards art- adopted, there are several smelters that may approach or meet primary and secondary ambient air quality standards because of location, meteorological conditions, sulfur content of the concentrate being smelted, and the smelting practice.
Status of Technology for Removing Sulfur Dioxide from Smelter Gases
The distinction between the two typos of air pollution control standards- air quality (or ambient air) and emission-are important considerations when examining the state of the art for controlling smelter gas discharge. The air quality standard permits alternatives for the smelter operator to meet the standard; the emission standard demands removal of a specified percentage of sulfur in the smelter feed.
In 1958 the Bureau of Mines published a compilation of information on the chemistry of sulfur dioxide, emphasizing reactions having application to the problem of air pollution. The publication also reviewed both existing and proposed technology for removing sulfur dioxide from industrial waste gases. Although the publication was oriented toward dilute gas streams from coal-burning powerplants, technology for treating richer gas streams, such as those from nonferrous smelting, also was reviewed.
In 1969, Arthur G. McKee & Co. published an evaluation of the problems existing in the nonferrous smelting industry for controlling sulfur dioxide air pollution. This report included a review of the technology for removing sulfur dioxide from smelter stack gases, and concluded that of the host of proposed processes, only five had been sufficiently tested to provide enough data so that costs could be estimated for application to a range of smelter conditions. The five processes were the contact sulfuric acid process; the Cominco absorption process; the Asarco process for producing elemental sulfur; the lime wet-scrubbing process; and the limestone wet- scrubbing process. A few other processes currently are being developed and may eventually prove superior to those cited by the Arthur G. McKee & Co. Of these new processes, the citrate absorption process, under development by the Bureau of Mines, appears attractive, especially for lean gas streams.
Processes tor removing sulfur oxides from gas streams are based on (1) conversion of SO2 in gas to SO3 for production of H2SO4, (2) separation and concentration of SO2, (3) reaction of SO2 to yield solid sulfur compounds, and (4) reduction of SO2 to elemental sulfur. Process selection will be dictated by economic conditions for sulfur byproduct disposal and by variation in the nature of the off gases, such as sulfur dioxide concentration, impurities carried over from smelting operations, and fluctuations in gas flow rates. Other factors, both economic and noneconomic, such as geographic location, nearness of markets for sulfurous products, available land for product disposal, and potential water pollution problems will influence the choice of method for controlling emissions.
Conversion of SO2 to SO3 for Acid Production
The contact sulfuric acid process is the only well-established chemical process for removing sulfur dioxide from smelter gases. Basic steps in the process are shown schematically in figure 11.
After the smelter gas has been cleaned of particulates, sulfur trioxide is removed in a mist Cottrell precipitator and the gas is dried with strong
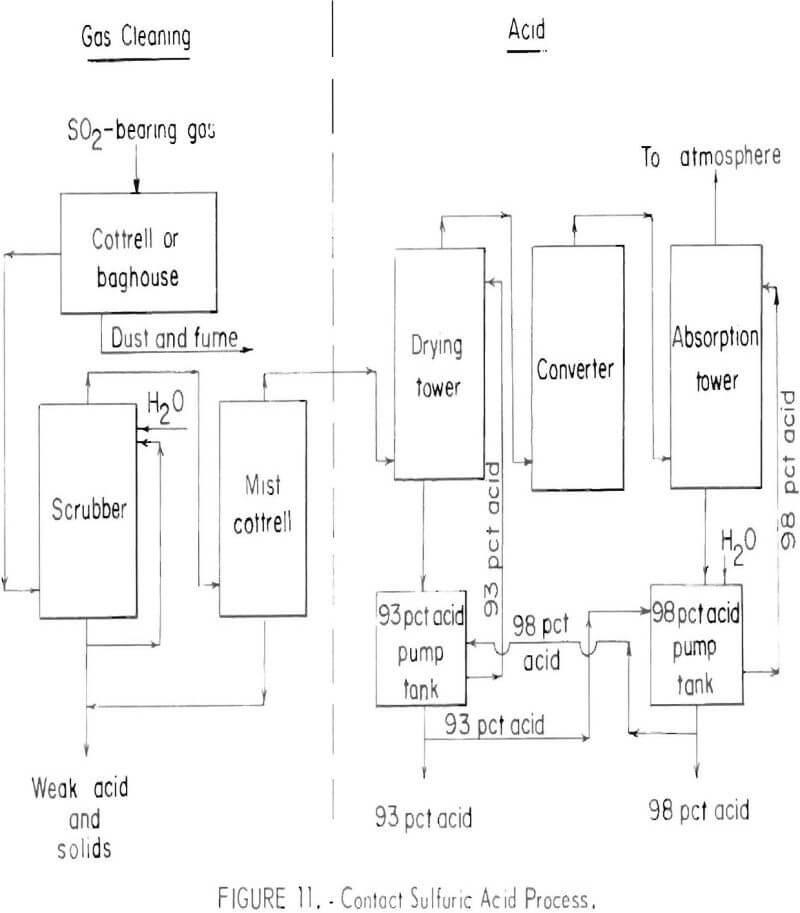
sulfuric acid. The sulfur dioxide in the dry gas is then oxidized to sulfur trioxide in a catalytic converter. Finally the sulfur trioxidc is absorbed in strong (98 percent) sulfuric acid to yield the product of the plant. The tail gas is treated to remove droplets of acid and normally is vented to the atmosphere. Tail gases will contain from 0.2 to 0.5 percent sulfur dioxide. In some cases the tail gas must be treated to lower the concentration of sulfur dioxide before discharging.
Strong gas is a prime consideration for acid production, although this is not a technological limitation, but a matter of economics. Acid plants can be designed to operate on gases containing only a fraction of a percent of sulfur dioxide, but they are too expensive to build and operate under normal practice. The normal economic minimum concentration of sulfur dioxide in acid plant feed gas is 3½ to 4 percent.
The major factor limiting production of sulfuric acid from smelter gas is marketability of the acid. Eighty-seven percent of the sulfur consumed in the United States during 1970 was converted to sulfuric acid, and about 90 percent of this was produced from Frasch sulfur. Most of the acid plants are located close to centers of sulfuric acid consumption in the eastern part of the United States. Over 90 percent of all sulfur discharged to the atmosphere as oxides from nonferrous operations is from smelters west of the Mississippi River, with copper smelters accounting for the major portion. Acid production to reduce sulfur dioxide emissions at the western smelters would be in excess of what could be internally consumed in company operations or sold at nearby markets. Disposal of the excess as a waste material would be necessary.
To safely dispose of sulfuric acid, it must first be neutralized with lime or limestone to produce gypsum, an inert compound. This gypsum must then be impounded. Neutralization of the acid entails a series of steps including mining, hauling, and grinding the limestone before it is mixed with the acid. The limestone must be finely ground, otherwise consumption is excessive owing to the formation of calcium sulfate which occludes the limestone particles. Because of the inefficient utilization of the limestone, about 2 to 3 pounds of limestone probably will be required for each pound of sulfuric acid that must be neutralized. If the limestone is converted to lime before neutralization, the added cost of calcining must be included.
Disposal of the neutralized acid poses serious problems for some smelters. If cheap land is not available, disposal could eliminate acid production as a control process for sulfur dioxide emissions. Moreover, a potential water pollution problem exists where overflow or seepage from the impoundment might occur. Either limestone or lime, which is produced from limestone, almost inevitably contains magnesium in amounts ranging from a few tenths of one percent to several percent. Neutralizing waste sulfuric acid with lime or limestone often creates water-soluble magnesium sulfate, which could contaminate local water supplies. The magnesium can be rendered insoluble by using excess lime, but it is questionable that excess limestone will give the same results.
Separation and Concentration of SO2
Because production of sulfuric acid will not solve the pollution problem for all smelters, several cyclic absorption systems have been developed and used for gas streams. The concentrated sulfur dioxide can be used as such, or converted to acid, or it can be reduced to elemental sulfur. The simplest procedure for regenerating the absorbent employs stripping with steam, but chemical regeneration methods are also used. The choice of absorbent is dependent upon the initial concentration of the sulfur dioxide in the gas stream. Absorbents that afford favorable equilibrium and capacity for removing sulfur dioxide from gas streams of high concentration do not work well with dilute gas streams. Absorbents favorable for dilute streams tend to require excessive amounts of steam for regeneration.
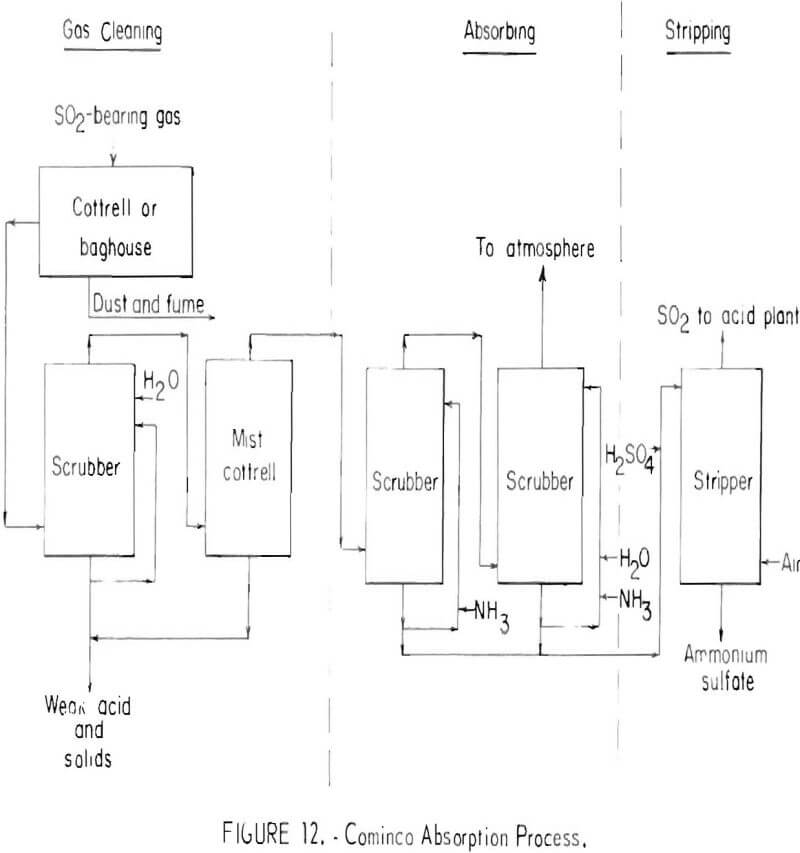
There are two principal regenerative absorption processes in commercial use for separating and concentrating sulfur dioxide from smelter gases. One uses a solution of ammonium sulfite-bisulfite as the absorbent; the other uses anhydrous dimethylaniline. Both have found only a limited application. Developed by the Consolidated Mining and Smelting Company of Canada, Ltd. (Cominco), the ammonium sulfite-bisulfite absorption process employs chemical regeneration with sulfuric acid to release sulfur dioxide and form ammonium sulfate. A simplified flowsheet for the process is shown in figure 12.
Sulfur dioxide-bearing gas, free of sulfur trioxide and particulates, is absorbed by an ammonium sulfite-ammonium bisulfite solution. The sulfur dioxide in the gas reacts with ammonium sulfite to form the bisulfite. Ammonia is added to convert part of bisulfite to sulfite and recycled to the absorption scrubbers. The remainder of the bisulfite solution is diverted to the stripper, acidified with sulfuric acid, and stripped with air to produce about a 25-percent sulfur dioxide gas and a solution of ammonium sulfate containing about 10 percent of the feed sulfur. The process will recover 90 percent of the sulfur dioxide from dilute flue gases, even at concentrations as low as 0.5 percent. Tail gases contain as little as 0.03 percent sulfur dioxide. A serious disadvantage of the process is the high cost of ammonia.
The Cominco absorption process has been used to treat zinc roaster gases containing as much as 6 percent sulfur dioxide, but its most important application has been in recovering sulfur dioxide at concentrations of 1 percent or less from lead sintering machine gases and from sulfuric acid plant tail gases. The economics of the process are largely dependent upon the existence of adequate markets for the ammonium sulfate produced and if no market exists, production and disposal costs must be viewed as additional smelting expense.
The dimethylaniline (DMA) absorption process developed by the American Smelting and Refining Company has been used for recovering sulfur dioxide from smelter gases containing from 4 to 10 percent sulfur dioxide. Basic steps of the process are shown in figure 13. After the gas is cleaned, the sulfur dioxide is absorbed by dimethylaniline in a bubble-cap tower which contains an absorption section, a soda scrubber and an acid scrubber. Tail gases from the DMA absorption section in the bottom of the tower are scrubbed with a dilute sodium carbonate solution in the middle section of the absorption tower, thereby recovering residual sulfur dioxide from the gas stream. However, the sodium carbonate serves the more fundamental purpose of neutralizing the sulfuric acid used for DMA vapor recovery as well as any acid formed through sulfur dioxide oxidation. Neutralization of this acid is essential to permit recovery of dimethylaniline which otherwise would be lost as water-soluble DMA sulfate in waste water discharged from the stripping column. In the upper section of the absorption column tail gases are scrubbed with dilute sulfuric acid to recover DMA vapor which would otherwise escape to the atmosphere.
The loaded DMA solution is stripped with steam in the stripper section of the stripping column. Dimethylaniline and sulfur dioxide are recovered from the combined aqueous scrubber solutions, by steam distillation in the lower section of the stripping tower. The hot gas stream leaving the stripper, containing sulfur dioxide, steam, and dimethylaniline vapor, is cooled in the upper or rectifier section of the stripping column. In the presence of the
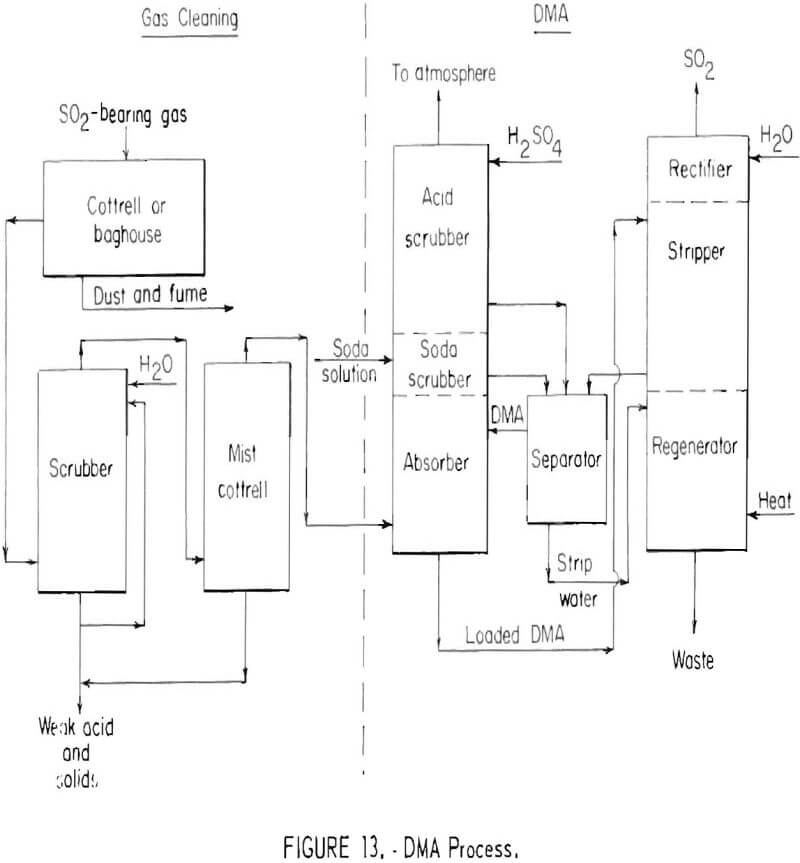
sulfur dioxide, dimethylaniline vapor is recovered as water soluble dimethyl-aniline sulfate. This leaves essentially pure sulfur dioxide which can be liquefied. The process can recover from 90 to 95 percent of the sulfur dioxide from a gas stream containing 2 to 4 percent sulfur dioxide, and as much as 95 to 98 percent from a gas stream containing 8 to 10 percent sulfur dioxide.
The equilibrium between dimethylaniline and sulfur dioxide is unfavorable for economic recovery of sulfur dioxide from gas streams containing less than 1.5 to 2.0 percent sulfur dioxide. The capacity of the solvent is good, however, when initial sulfur dioxide concentrations arc high. In the United States, the DMA process has found limited use for production of liquid sulfur dioxide from lead and copper smelter gases.
Another process proposed for smelters to concentrate sulfur dioxide is the Wellman-Lord process. In the absorber section the gas is contacted with a sodium sulfite-sodium bisulfite solution which absorbs the sulfur dioxide to form additional bisulfite. The enriched solution is then boiled by indirect steam heating in an evaporator-crystallizer to decompose the sodium bisulfite solution into a wet gas of sulfur dioxide and steam and a precipitate of sodium sulfite crystals. The resulting slurry containing the precipitates is centrifuged. The solids subsequently are dissolved in water for recycling. Part of the liquor from the centrifuge is returned to the evaporator for solids-density control. The gas passes through a condenser to concentrate the sulfur dioxide. This process currently is being used on the tail gases of the Olin Corp. sulfuric acid plant at Paulsboro, N.J. However, application of the Wellman-Lord process to smelter gas is only speculative and cost estimates recently published are discouraging.
A variation of reacting sulfur dioxide with an absorbent has been developed by the Chemical Construction Corp. and Basic, Inc. In this process, a slurry of magnesium oxide is used to remove sulfur dioxide from gas streams in a venturi scrubber. The magnesium sulfite product is then decomposed thermally to yield concentrated sulfur dioxide for acid production and magnesium oxide for recycling. A pilot plant to test the absorption and decomposition steps of this process is being constructed at Boston Edison’s Mystic Station No. 6 power plant. No data are available to evaluate application of the process to smelter gases.
The market for sulfur dioxide as an end product is limited, accounting for only 1 percent of the domestic consumption of sulfur. Unless new markets are developed, processes that separate and concentrate sulfur dioxide must be considered as preliminary steps to obtain suitable feed for the production of acid, elemental sulfur, or inert sulfur compounds for disposal.
The Cominco and Asarco DMA processes have found limited application but have not been demonstrated as viable processes for treating large volumes of dilute smelter gas. It is doubtful that either could be developed and engineered to a scale for implementation at an operating smelter in less than 3 years. The fruition of other processes that separate and concentrate sulfur dioxide will be several years later, perhaps as long as 5 to 10 years from now.
Reaction of SO2 to Yield Solid Sulfur Compounds
Typical of the processes for reacting the sulfur dioxide gas streams to form solid sulfur compounds are the lime and limestone wet-scrubbing processes that yield a waste product, as shown in figure 14. In the lime wet-scrubbing process, burnt lime obtained from limestone is slaked and the slurry is used to absorb sulfur dioxide from the gas stream. Calcium sulfate and calcium sulfite are formed and are separated by thickening into a sludge which must be impounded. In limestone wet-scrubbing, finely ground limestone is used instead of lime. Limestone is not as efficient as lime for scrubbing and, in addition, it may not be suitable for gases deficient in oxygen. It has been suggested that a high recovery of sulfur dioxide might be obtained by operating the two scrubbing systems in series; the tail gas from limestone scrubbing would be treated by a lime or caustic scrubber before discharging. A good grade of limestone will be required, and substantial costs will be entailed in mining, hauling, grinding, and calcining. Disposal of the final product will entail problems similar to those previously mentioned for disposing of neutralized acid. For smelters located in desert regions, water losses by evaporation may limit application of wet-scrubbers.
In recent years both lime and limestone wet- and dry-scrubbing have been investigated for removing sulfur oxides from the stacks of power plants burning high-sulfur coal. Troubles encountered have included buildup of gypsum deposits, which causes plugging in various parts of the systems, low sulfur dioxide removal, and difficulty in collecting particulate matter from the exit gas.
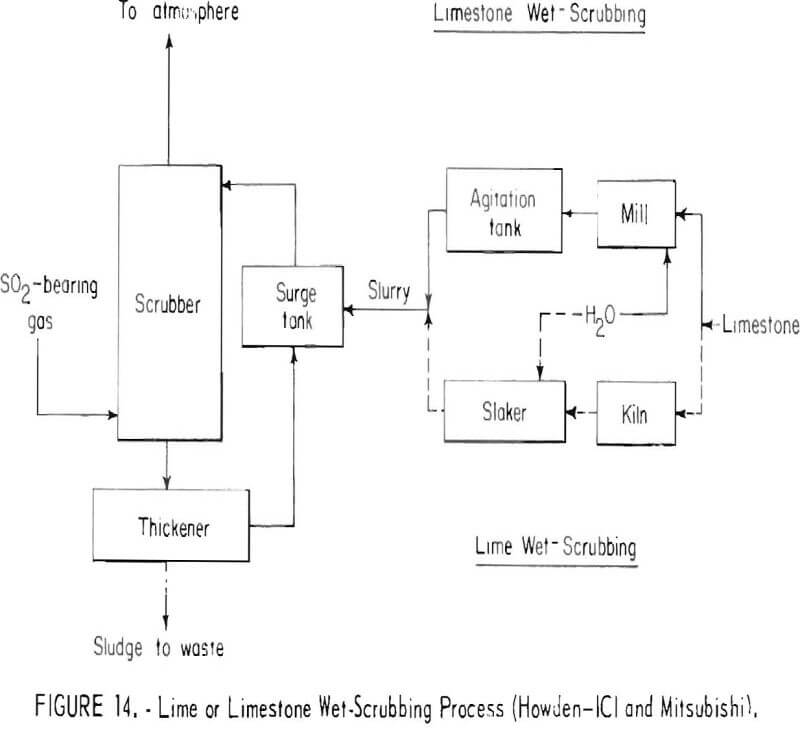
In spite of the difficulties encountered, the lime and limestone wet-scrubbing processes are flexible and applicable to variable gas flows and sulfur dioxide concentrations. They may well be the first processes applied to dilute smelter gases and could conceivably be on-stream within 5 years. The Smelter Control Research Association, Inc., is currently evaluating the application of limestone scrubbing of copper smelter gases at a western smelter.
Reduction of SO2 to Elemental Sulfur
Reduction of sulfur dioxide to elemental sulfur has been investigated at a number of smelters. Typical of the reduction processes is the American Smelting and Refining Company’s sulfur or brimstone process shown in figure 15, which was developed to a semi-commercial scale in the early 1940’s. In this process, smelter gases are cooled, cleaned, and reacted by combustion with natural gas (CH4) to generate a flame temperature of approximately 2,300° F. In the combustion stage of the process, oxygen in the gas stream is consumed and natural gas reacts with the sulfur dioxide to yield a gaseous mixture containing sulfur vapor, hydrogen sulfide (H2S) carbonyl sulfide (COS), residual sulfur dioxide, carbon dioxide, water vapor, and nitrogen. After cooling the gases to approximately 840° F, carbonyl sulfide and part of the
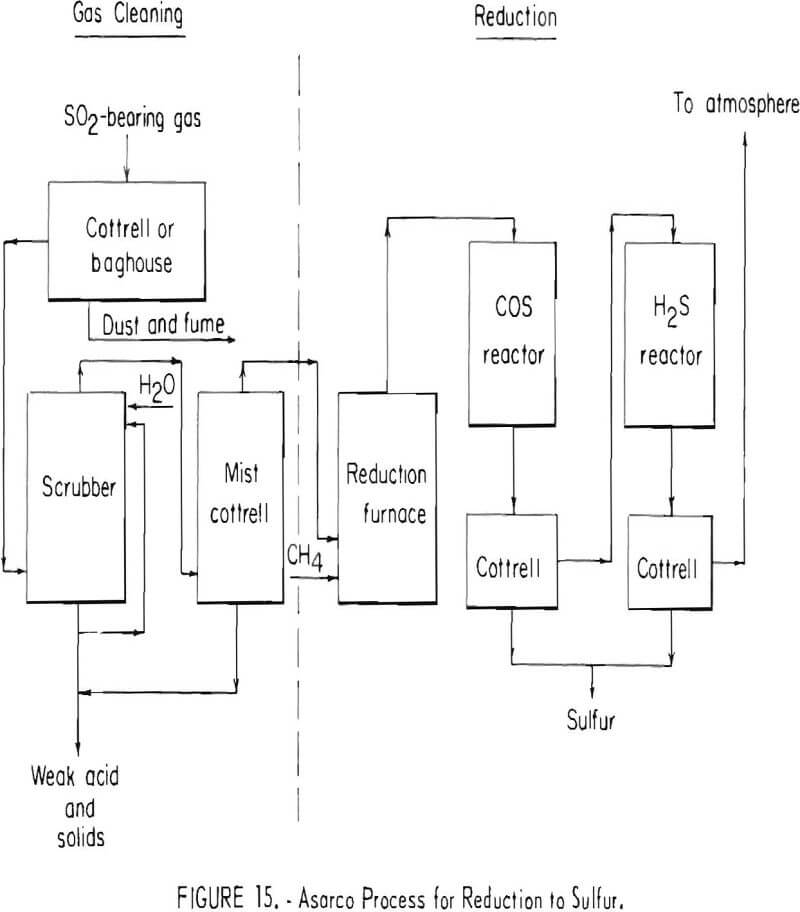
residual sulfur dioxide are reacted in the presence of a bauxite catalyst to yield additional sulfur vapor. Gases from the carbonyl sulfide converter are cooled to condense the sulfur vapor and the resultant liquid sulfur is collected in an electrostatic precipitator. Sulfur-bearing gases present in the gas stream leaving the precipitator are principally hydrogen sulfide and sulfur dioxide in a volume ratio of 2 to 1. After reheating to about 400° F, the gases are passed through an activated alumina catalyst to react the hydrogen sulfide and sulfur dioxide to form additional sulfur vapor. The sulfur formed by this reaction is cooled to about 250° F and then is collected in a second electrostatic precipitator.
The process was applied to gases containing from 5 to 7 percent sulfur dioxide. Actual pilot plant recovery of elemental sulfur was approximately 90 percent. Through provision of a second stage hydrogen sulfide converter operated at a temperature somewhat lower than that of the single stage hydrogen sulfide converter of the pilot plant, it is estimated that sulfur recovery could have been increased to approximately 95 percent.
The Asarco brimstone process, as conceived and operated 25 to 30 years ago, was complicated and expensive. A much simplified and improved version of the process is now being investigated jointly by two copper companies with a test unit having a capacity of 20 tons of sulfur per day. Information available to the Bureau of Mines indicates at least 2 more years of development work is needed before the process can be demonstrated successfully.
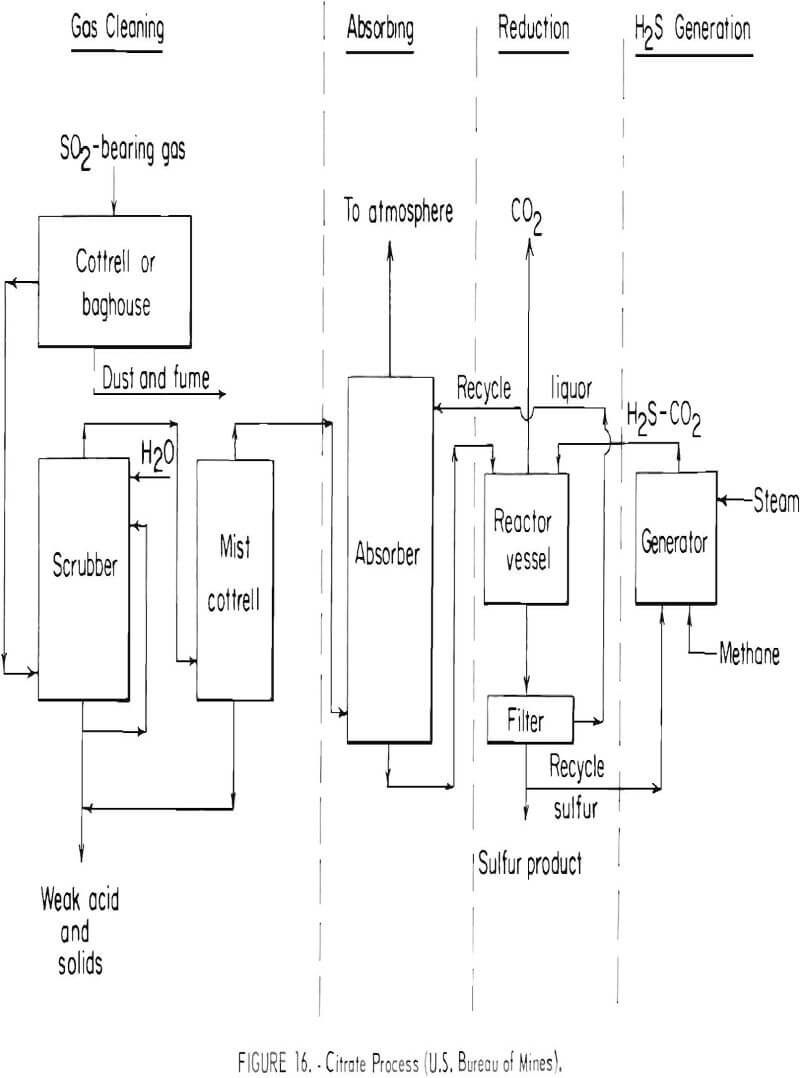
The Bureau of Mines currently is developing a method for recovering and producing elemental sulfur from stack gases. The method is referred to as the citrate process and is shown schematically in figure 16. The gas is scrubbed with an aqueous solution of sodium citrate and citric acid, which absorbs the sulfur dioxide. Hydrogen sulfide gas is then introduced into the solution where it reacts with the absorbed sulfur dioxide to form elemental sulfur, which is easily filtered from the solution. Part of the sulfur that is produced is reacted with natural gas (methane) to form the required hydrogen sulfide needed for the sulfur dioxide reaction step. The process was tested in a small unit having a gas flow capacity of 350 cubic feet per minute at the Magma Copper Co., San Manuel, Ariz., smelter. Test results from this unit indicate that the sulfur dioxide can be removed from lean gas streams satisfactorily and that the sulfur is readily recoverable. Over 90 percent of the sulfur was recovered from reverberatory furnace gas containing about 1.5 percent sulfur dioxide. The data obtained during the test indicated that the capital and operating costs of a large plant would increase the cost of producing copper by 2 to 3 cents per pound of copper smelted with no credit for sale of sulfur. It is estimated that a plant using this process could be operable in 4 to 5 years. The process has not been evaluated for lead and zinc smelter gases.
Approaches to Solving the Problem
Individually, every primary copper producer and several lead and zinc producers have implemented programs that will lead to at least partial compliance to both air quality and sulfur emission standards. These programs vary according to smelter age and location, capability to consume or market acid, sulfur content of the feed, and smelting practice. Companies operating old smelters are faced initially with the problem of collecting and channeling their off gases into a flue system for subsequent treatment. This step is essential for compliance with stringent emission standards, and poses costly and time-consuming modifications.
For some smelters, both old and relatively new, different charging practices will need to be developed. For some, only installation of new furnaces will give the required high-sulfur off gases. Announcements have been publicized for replacement of reverberatory furnaces with flash-smelting or electric smelting equipment to produce matte and an off gas rich enough in sulfurous oxides for the production of acid.
Almost every smelter operator is either planning or already constructing new capacity for making sulfuric acid as an approach to reducing smelter emissions. It is realized, however, that this is only a partial solution since neutralization and disposal of excess acid introduces other problems. The construction of an acid-making facility involves more than the addition of a conventional acid plant. In most cases the cost of the plant will be exceeded by the expense of installing new water-cooled hoods on the converters or the other gas-collecting systems, tightening up or installing new flues and duct-work, and providing extensive gas-cleaning equipment. For example, one copper company has spent $17 million for a 1,000-ton-per-day acid plant to remove from 50 to 60 percent of the smelter’s sulfur dioxide emission. Of this amount, almost two-thirds was attributed to gas collection and cleaning equipment. A company producing lead and zinc at a common site recently completed a program that allows conversion of 80 percent of the sulfur to acid. This program was started in 1954, and reportedly cost $20 million, of which $6½ million was spent in the last 2 years, equivalent to the company’s net income for that period.
Even though acid production is a proven technology for removing sulfur dioxide from the richer smelter gases, the installation of acid plants will require considerable time. This time will range from 2 to 3 years for the modern smelter with favorable conditions and possibly longer for old smelters where considerable gas collection and cleaning equipment must be installed. Moreover, the pace for constructing the treatment facilities will be slow if serious curtailment in production is to be avoided.
Some smelter operators are seeking solution to the problem through developing programs that, if successful, will allow production of either liquid sulfur dioxide or elemental sulfur, in addition to sulfuric acid. It is not likely that this developing technology will be commercially installed in less than 5 years, and for some, 10 years might be required before satisfactory plants are on-stream.
As mentioned before in the discussion of closed-loop control, several producers are monitoring both weather patterns and atmospheric sulfur dioxide concentrations for the area affected by the smelter. Through continuous evaluation of the data, the smelter operation is adjusted to hold sulfur oxide emissions at a level meeting State ambient air quality standards.
A possible alternative to solving the smelter air pollution problem is to recover the metals by methods other than the pyrometallurgical reduction process. Many hydrometallurgical and other chemical processes have been proposed some have been intensively investigated, and a few have been practiced. Acid leaching of sulfide ores too low in grade to justify milling costs and oxide minerals that cannot be concentrated is the only hydrometallurgical process that makes a significant contribution to domestic copper production. The amount of copper recovered by leaching has increased more than threefold in the last 25 years, but still accounts for less than 15 percent of the primary metal produced. However, except for leaching, the nonsmelting methods for copper have been either too costly to compete against the smelter or lacking in sufficient technology for engineering full-scale plants. Matte smelting and converting will continue as the principal process for copper production during the remainder of this century, as will the pyrometallurgy process for recovering lead and zinc. Nonsmelting, pollution-free processes will not emerge as an alternative to emission control for many years as ready substitutes for smelting. A strong and continuing research effort is needed to develop the technology, and this must be followed by demonstration on a scale that produces meaningful data for both engineering design and cost evaluation. Accordingly, eight copper producers that account for substantially all of the primary copper production have established a nonprofit research organization to develop new or improved methods for removing sulfur dioxide and particulate matter from smelter stack gases. The organization, called Smelter Control Research Association, Inc., will conduct research and sponsor investigations to test selected control processes at member plants.
The technology for controlling sulfur oxide emissions in the lead and zinc industries is well established. Updraft sintering of lead concentrates for lead and several roasting processes for zinc are capable of capturing enough of the sulfur dioxide to meet a 10-percent or possibly even a 7-percent emission standard. In some plants this will require extensive changes in sintering and roasting plants, ducts, the gas-cleaning system, and installation of an acid plant. The only question that arises is whether some of the smelter operators are prepared to risk the capital required to install new acid facilities and make the necessary plant modifications. In light of the extreme competitiveness of lead and zinc in world markets, and the slim margin of profit on these metals, the increased cost of compliance will be difficult to pass on and may have to be absorbed by the companies.
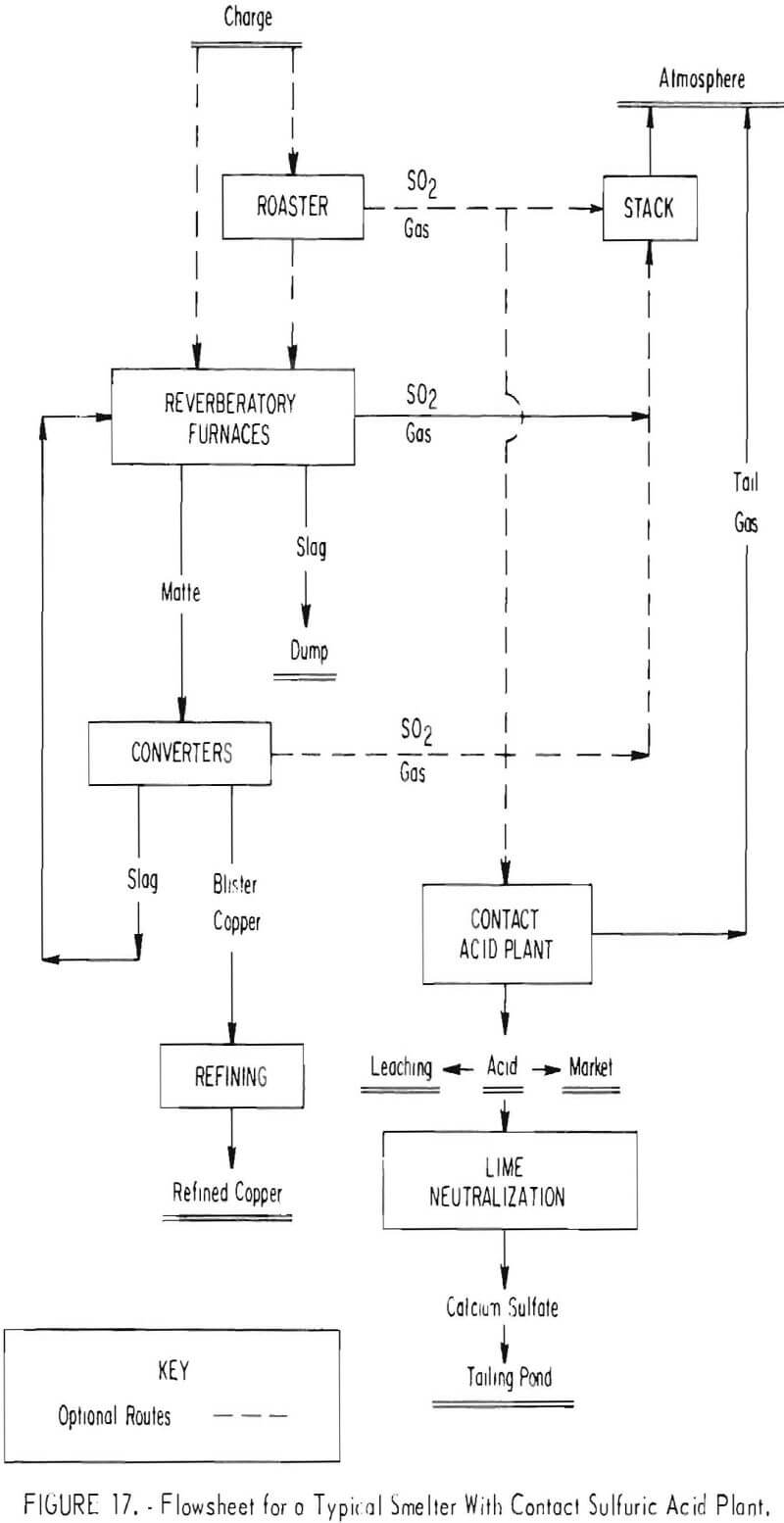
Unlike the well-defined technology available to the lead and zinc industry for capturing the smelter off gases, the alternative choices open to the copper companies are varied and much more complex. The copper companies are proposing several different ways in which they intend to capture sulfur dioxide from their smelter gases, either to comply with the Federal primary and secondary ambient air quality standards or the 10-percent emission standard that has already been imposed by several States. Diagrammatic flowsheets embracing alternative approaches of proven or developing technology which have been proposed for meeting the needs of individual smelters in complying with the standards are shown in figures 17 through 21. The simplest flowsheet (fig. 17) is typical of current smelter operations which either have no sulfur oxide recovery systems or employ the only recognized proven technology (contact sulfuric acid plant) to achieve partial control of the sulfur oxides.
Without an acid plant all gases generated in the smelter are discharged in the atmosphere. Green-charge smelting and acid production from the gas captured from tightly hooded converters can recover about one-half of the sulfur dioxide generated in the smelter, which is enough to meet Federal primary and secondary ambient air quality standards. Depending upon the size of the acid plant, at least an $18 to $25 million capital investment is necessary to install the proper water-cooled converter hoods, waste-heat boilers for cooling the converter gas, gastight balloon flues, hot gas precipitators, ducts, gas-wet scrubbers, and an acid plant to achieve this level of sulfur oxide control.
Incorporating a roaster, such as a fluidized-bed reactor, to produce a strong gas which can be combined with converter off gas for acid manufacture, possibly can approach 85- to 90-percent sulfur oxide removal. The best commercial operation to date employing this system has achieved only 60- to 65-percent sulfur oxide removal, because of inadequately hooded converters and operating difficulties in roasting.
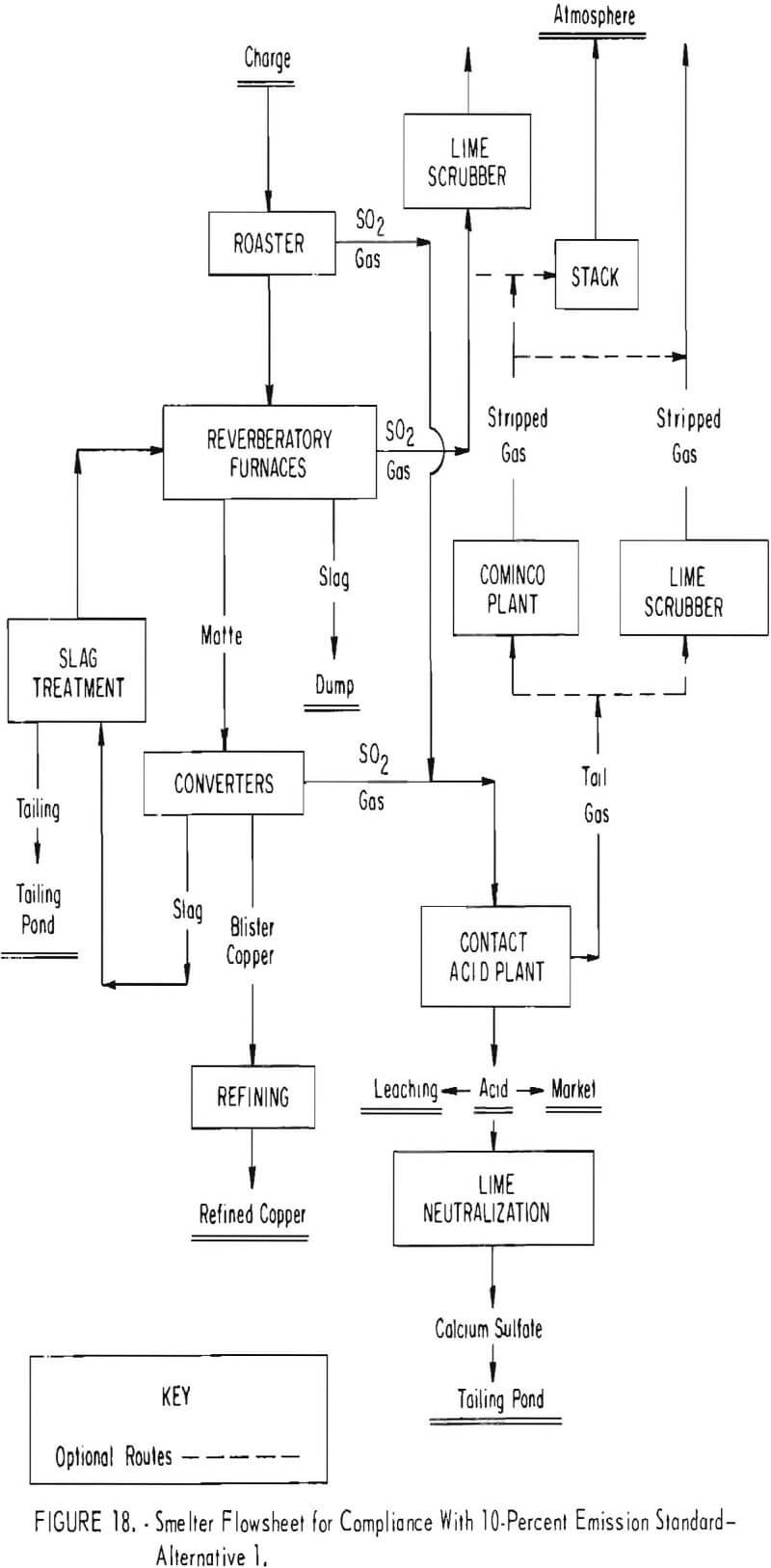
Figure 18 shows a proposed smelter plant flowsheet designed to meet the 10-percent emission standard. The proposed plant will employ fluidized-bed roasting of the charge, controlled reverberatory furnace operation to minimize sulfur oxide generation at that point, special converter slag treatment, and a tightly hooded converter and duct system. Sulfuric acid will be produced from the combined gas. It is thought that by using lime-scrubbing of the acid plant tail gases, the 10-percent emission standard may be met. Should the lime-scrubbing technology, which still is in a state of development, prove unfeasible, the Cominco process will be considered for removing sulfur oxides from the acid plant tail gases. However, even with these controls, there is a likelihood that lime-scrubbing or some other technology as of yet not fully developed might be needed to scrub reverberatory gases to assure compliance with the 10-percent standard. The cost of such a system, including roasting, is estimated at $40 to $45 million. This figure relates to a relatively new smelter and conceivably could run considerably more if incorporated in an old smelter.
A second alternative proposed for capturing the sulfur oxide gas to achieve a 10-percent emission standard is shown in figure 19. Key to this technology is use of dimethylaniline to absorb and concentrate the weak gases generated in the reverberatory furnace, and during low cycles of converting. The gas absorbed in the DMA process is regenerated as liquid sulfur dioxide for direct marketing, as a concentrated gas for feed to a contact sulfuric acid plant, or as feed gas to an elemental sulfur plant.
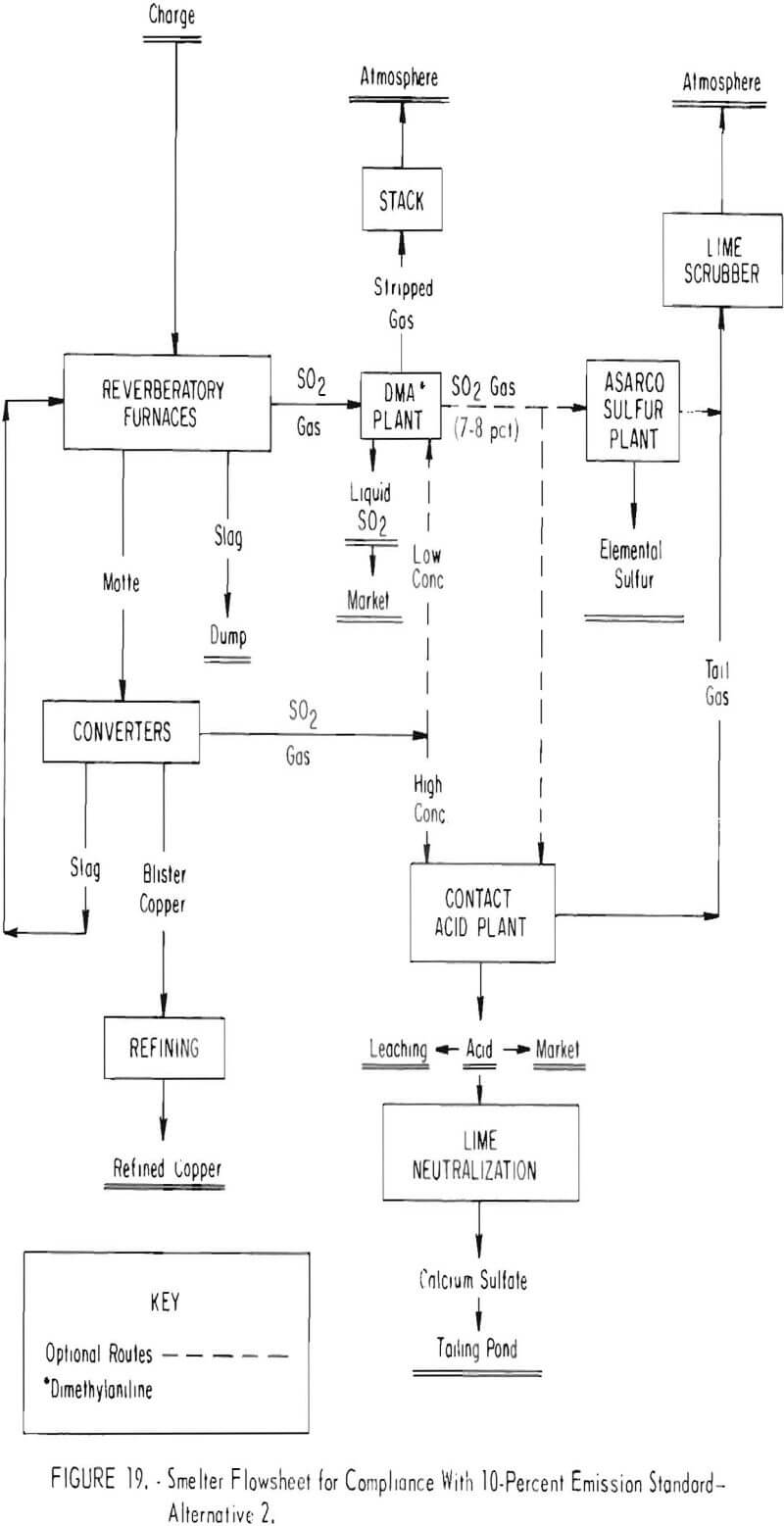
The system planned for several smelters will involve absorbing the sulfur oxide gases from the reverberatory furnaces in a DMA plant on a continuous basis. Strong gas from the converters will be fed directly to a contact sulfuric acid plant except during periods of weak gas generation in the converters. During this period, the gas will be diverted to the DMA plant where continuous bleed off of concentrated sulfur dioxide will assure a uniform concentration to the acid plant. Essentially the same procedure will be used to produce elemental sulfur instead of sulfuric acid, or the concurrent production of acid and sulfur.
Success of various systems in meeting 10-percent emission standards will depend upon applying technology available for the DMA process which heretofore has not been used for concentrating copper smelter gas. In addition, substantial modification and rebuilding of plant facilities and equipment will be necessary. Typical changes that will be required are construction of new or modification of existing reverberatory furnaces and charging systems to assure off gases containing a minimum of 1.5 percent sulfur dioxide; installation of waste-heat boilers, new hot gas precipitators, tighter flues, and converter modifications including tight water-cooled hoods; erection of the DMA, sulfur, and contact acid plants; and the addition of ancillary equipment such as lime-scrubbers, acid neutralization systems, and liquid sulfur dioxide storage facilities. One company has estimated that the capital cost of modifying its Arizona smelter to recover the sulfur dioxide as elemental sulfur and sulfuric acid will range from $117 to $132 million. By comparison, the company estimates that the cost of plant modifications which would capture enough sulfur dioxide from the smelter gas streams to assure compliance with Federal ambient air quality standards would only be $30 million.
Another proposed installation to capture 90 percent of the sulfur in the feed will produce sulfuric acid and liquid sulfur dioxide. The capital costs estimated by the company for this installation will range between $50 and $60 million. By comparison, their estimated costs for removing enough of the smelter off gases to meet ambient air quality standards would be approximately $17 million.
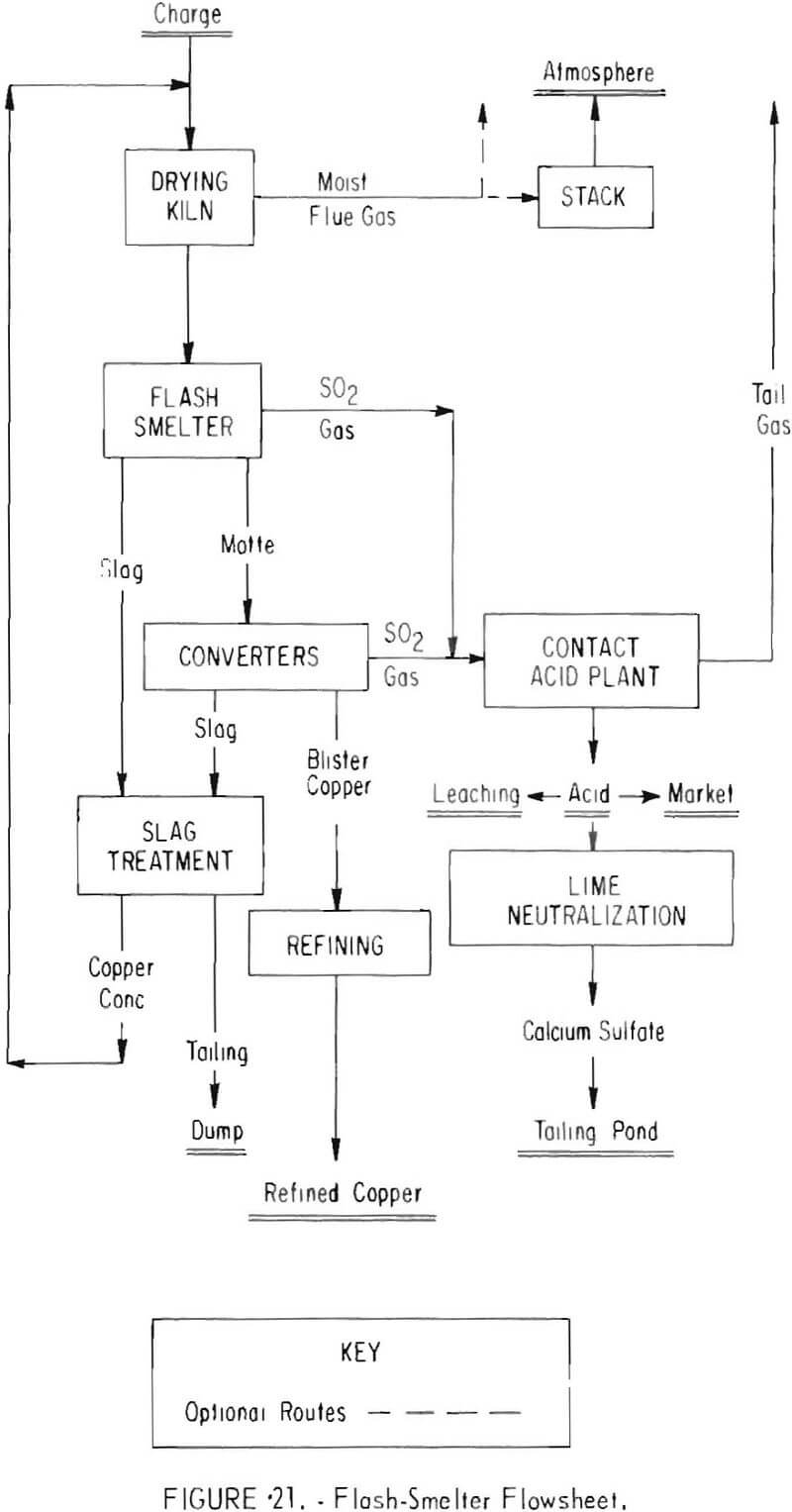
Two other smelting systems designed to meet 10-percent emission standards are shown in figures 20 and 21. In these instances, electric furnaces (fig. 20) and flash smelters (fig. 21) replace the reverberatory furnaces currently used.
The electric furnace made by the Boliden Co., Sweden, will provide for a controlled volume and grade of off gas (4 to 8 percent SO2) in the matte-smelting step. This off gas will be combined with converter gas for feed to the contact acid plant. Gas collecting and cleaning facilities, as well as airtight flues and ducts, will be required with the electric furnace to provide a gas suitable for conversion to add. Similarly, tightly hooded converters, equipped with waste-heat boilers, and the usual gas wet-scrubbing and cleaning facilities in conjunction with the contact acid plant will have to be installed. Although the electric furnace will handle a green charge, in the interest of power savings and better control of furnace operations the furnace feed will be dried.
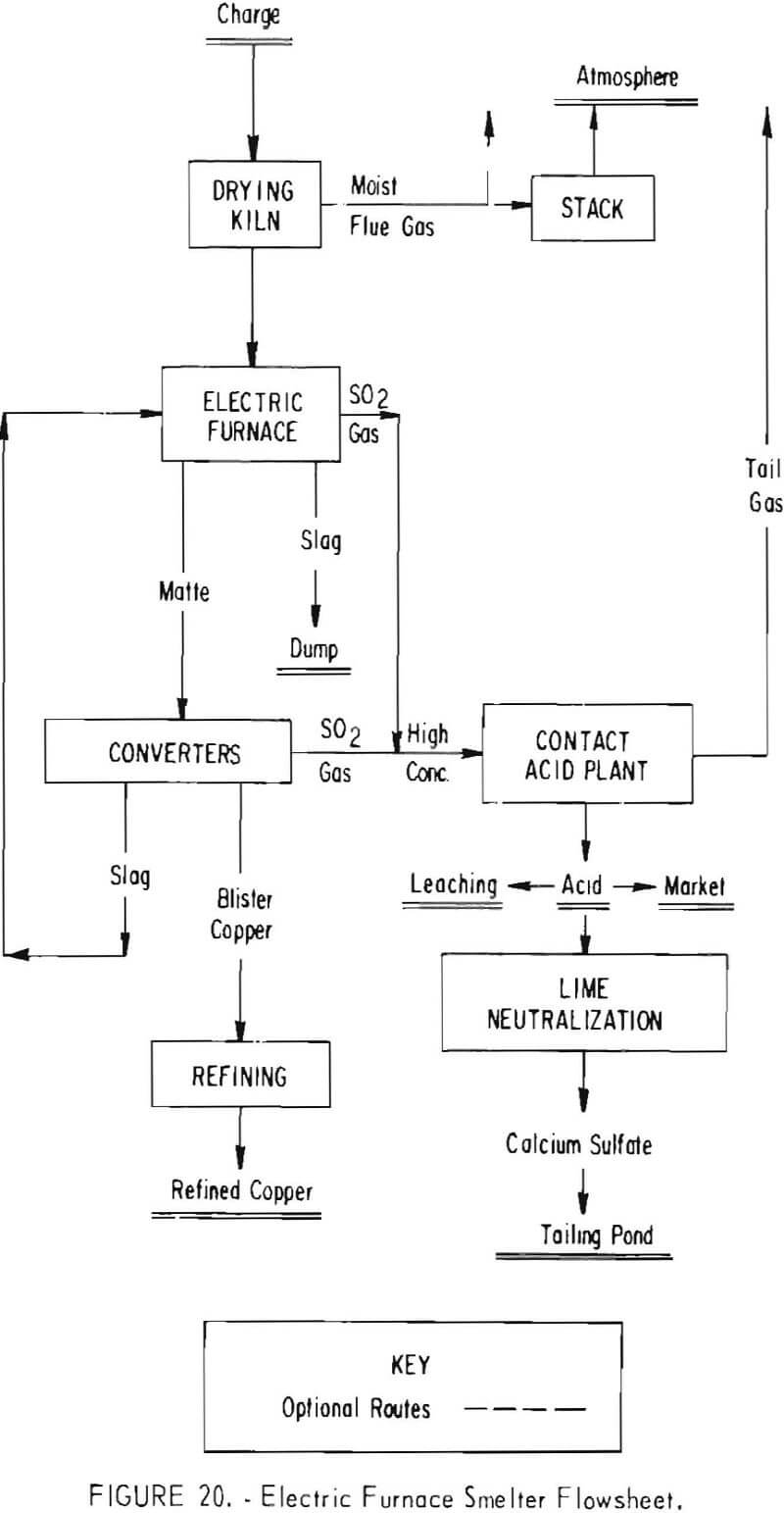
The estimated capital cost of the modifications incorporating the electric furnace system in one smelter, which will have an annual copper production capacity of 300,000 tons and an 1,800- to 2,000-ton-per-day sulfuric acid facility, is $75 million, of which $53 million is directly attributable to air pollution control. Another smelter incorporating this system, having a 110,000-ton annual copper production capacity with a 750-ton-per-day sulfuric acid plant, is estimated to cost about $28 million.
One domestic copper company undergoing an expansion program will replace its reverberatory furnaces with two flash smelters (fig. 21) developed by Outokumpu Oy, Harjavalta, Finland. The flash smelters are expected to produce a 10- to 14-percent sulfur dioxide gas which will be combined with the converter gas for conversion to acid. Salient features of the plant will be concentrate drying and slag-treatment facilities. Slag produced by the flash smelting process contains about 1 percent copper, and a composite with the converter slag contains 2.5 to 3 percent copper. The combined slag will be beneficiated by flotation to recover the copper in a concentrate assaying 20 to 25 percent copper that can be returned to the furnace. The flotation tailing is expected to contain about 0.3 percent copper. When completed, the smelter will have an annual capacity of 280,000 tons of copper and a 2,800-ton-per-day sulfuric acid plant. Estimated total cost of the program is $75 million.
Sulfur Products
Depending upon the technologies and processes adopted for removing sulfur oxides from smelter gases, the products obtained will be sulfur, sulfuric acid, concentrated sulfur dioxide, ammonium sulfate, or solid sulfur-bearing compounds having little market value. As stated earlier, sulfuric acid will be the major product because technology for its production is well-established. Disposition of these products will to some extent influence the choice of sulfur dioxide control schemes or processes that are ultimately incorporated in the smelter flowsheets.
From the smelting industry’s point of view, marketing of the byproducts, particularly sulfur or sulfuric acid, to offset at least part of the control costs is most desirable, and there probably will be keen competition among the sulfur-sulfuric acid producers and the smelting industry for markets for these commodities in areas where smelters are located. Furthermore, production of sulfur, sulfuric acid, and sulfur products from smelter gases will have a far-reaching effect on the national sulfur oversupply situation. Sulfur now is being recovered from sour natural gas in increasing amounts both in the United States and Canada. Even higher production can be expected in the future. Desulfurization of residual petroleum stocks and products is on the increase and the removal of sulfur or sulfuric acid from fossil fuel-fired electric power generating plants and industrial coal or oil burning plants can be expected to begin within a few years.
The potential production of Frasch and byproduct sulfur is shown in table 6. Domestic demand for sulfur in all forms in 1970 was slightly more than 9 million long tons, about 25 percent of which was byproduct sulfur. Sulfur present in the coal and petroleum alone, had it been recovered, was more than adequate to meet demand. This situation is not expected to change in future years.
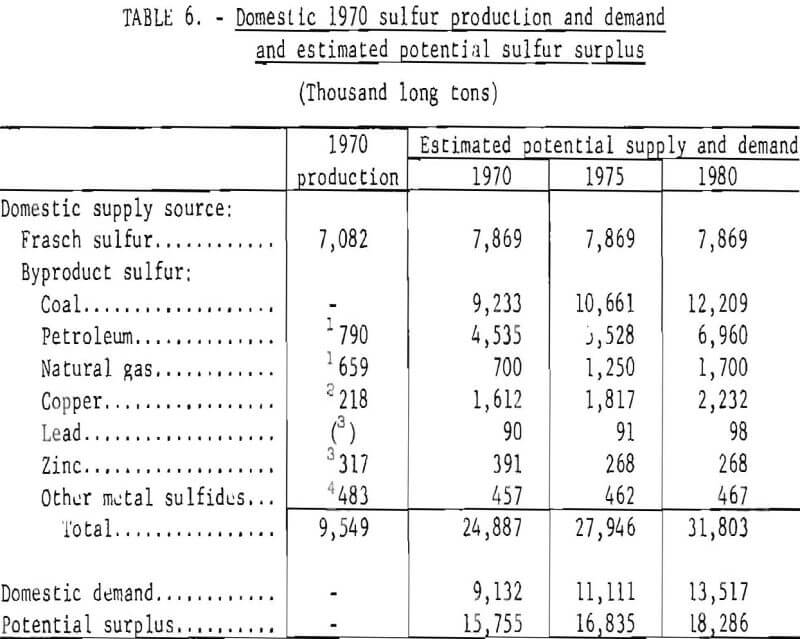
Large-tonnage sulfur production from the potential byproduct supply probably will not pose a serious problem in the short run. Production will be limited by the lack of suitable technology for recovering useful sulfur products from coal or flue gases. In the long run, however, this situation may well change as the industries producing or consuming coal or petroleum begin to remove sulfur or sulfur products either from the raw materials or from flue gases in compliance with Federal and State air pollution laws. It is not inconceivable that at that time the entire supply-demand structure of the domestic sulfur industry could be drastically altered by the dumping of large tonnages of sulfur or its compounds on the market. The demise of the Frasch sulfur industries and associated marketing might be one consequence of these actions.
In the nationwide picture, sulfur and particularly sulfuric acid recovered by treating smelter gas will be at a distinct competitive disadvantage because of the transportation costs to the major markets. Uses include fertilizer production, paper manufacture, cellulose fiber manufacture, and petroleum refining, the plants for which for the most part are located in areas remote from the smelters. Sulfur, sulfur dioxide, or sulfuric acid production in excess of local demand will have to be disposed of in some environmentally acceptable manner. Under these circumstances, removal of the sulfur oxides from the smelter gases as elemental sulfur will be advantageous. Sulfur can be shipped farther economically or it can be stored readily without air or water pollution and only minor land pollution. If needed at a later time, it can be recovered easily, and shipped without any unusual precautions. In either case, there is a good possibility that a substantial quantity of the sulfur removed from smelter gases will have to be discarded as a waste product.
Cost of Compliance
Only through a fortuitous set of circumstances will a smelter operator be able to realize a credit in his ledger for controlling sulfur dioxide emissions. For most companies, large capital investments for control facilities will be required which afford little opportunity for return on investment. These costs, combined with an increase in operating costs, will have to be balanced by an increase in the price of domestically produced metal or absorbed by the companies. Consequently, the magnitude of the cost is of serious concern to both industry and Government. In addition, the time allotted for compliance with the new environmental standards is forcing the companies to alter smelting practices and to install new processes and equipment, some of which have not been demonstrated on a commercial scale. Hence, within a few years the industry may be faced with the problem of operating with obsolete, outmoded, or costly noncompetitive methods.
Estimates have been made of the investment and operating costs that will be incurred by copper companies to achieve compliance with the air pollution standards for sulfur dioxide emissions. An early study made for the Environmental Protection Agency based on 1969 cost data and covering nine copper smelters representing approximately 60 percent of the domestic smelting capacity resulted in a capital cost estimate of $87 million. With regard to these cost estimates, the Environmental Protection Agency in a communication to the Bureau of Mines has pointed out that when their earlier estimate is updated and expanded to cover the total copper industry production, and to provide for other facilities such as site preparation and flue ducting, their current cost estimates are in the range of $250 to $270 million for capital investment and between $60 million and $80 million for annualized costs exclusive of any byproduct credit or disposal costs. These estimates are only for the cost to control air pollution and do not consider plant replacement needs that are essential to produce gas of sufficient sulfur dioxide concentration to be amenable to subsequent treatment by the proposed processes.
The Fluor Utah Engineers and Constructors Corp. was engaged in 1970 by the Kennecott Copper Corp. to determine the costs to the copper industry of conformance with the existing and anticipated air quality regulations. The study included 12 of the 14 western smelters. To avoid legal difficulties, Fluor Utah wrote the report on an industrywide basis using published information only, and does not reveal optimum action for any particular smelter. The mathematical model that was used, however, took into consideration variables in conditions at each smelter. Both capital costs and incremental costs were estimated for several different methods of controlling smelter emissions. Capital cost estimates ranged from a high of $607 million to a low of $264 million, and incremental cost estimates ranged from 16.6 to 2.8 cents per pound of copper produced. The five methods reported for controlling sulfur dioxide emissions were all considered capable of allowing the smelters to meet the primary Federal ambient air quality standard with some degree of production curtailment when necessary. Only two of the methods, caustic scrubbing in series with limestone scrubbing and caustic scrubbing with sodium sulfate production, were reported as capable of removing adequate sulfur dioxide to satisfy the 10-percent emission standard.
The $607 million capital cost estimate was for installation of acid plants and extensive production curtailment as a method of control. About one-half of the cost was for new smelters having a total daily copper capacity of 2,500 tons. The new smelter capacity was necessary to offset the loss of copper production during periods of severe curtailment at the existing smelters because the Fluor study model assumed a constant level of production. This method of control was one of two methods considered as proven technology, defined as systems that have been developed to the extent that they could be put into operation within 3 years. Incremental costs were estimated at 5.2 cents per pound of copper produced. It is questionable that the industry would be willing to continue the operation of those existing smelters where severe curtailment would be required. Moreover, it is doubtful that the industry would be willing to invest in installing the new capacity required to offset production loss during curtailment.
The other method considered as proven technology was the replacement of reverberatory furnaces with flash-smelting units and installation of acid plants for removing the sulfur dioxide. Capital costs estimated for this technology were $572 million and incremental costs of 2.8 cents per pound of copper produced.
The methods reported as second-level technologies, defined as those not yet developed to the point that they could be built in 3 years, were ammonium sulfate production, production of elemental sulfur via gas concentration and reduction, caustic scrubbing, and limestone scrubbing. Estimates were reported for three systems.
Capital costs for limestone scrubbers were the lowest at $264 million. The incremental costs were 3.8 cents per pound of copper produced. Capital costs for caustic scrubbers in series with limestone scrubbers were slightly higher, $275 million, and the incremental costs were 5.6 cents per pound of copper. The highest incremental cost reported, 16.6 cents per pound of copper, was for caustic scrubbers and sodium sulfate production. For this system, the capital costs were $331 million.
The Bureau of Mines has surveyed the copper smelting industry’s plans for meeting existing standards and has examined the individual cost estimates to implement these plans for most of the companies. The survey revealed proposed combinations of new reverberatory furnaces, new converter hoods, electric furnaces, flash-smelting units, DMA sulfur dioxide concentrators, acid plants, sulfur dioxide reduction plants, lime- and limestone-scrubbers, and acid neutralizing plants. The proposed combinations were for a variety of control schemes, each suited to the particular plant where it will be applied.
For 14 smelters operated by seven companies, these representing 98 percent of domestic capacity for primary copper, the total capital cost was estimated by the companies to be $598 million. Combined with estimated operating costs, the average increase in the cost of smelting copper would be about 4 cents per pound. The individual estimates for the seven companies are listed in table 7. Except for two of the smelters, the cost estimates are for control measures that will meet the 10-percent sulfur emission standard. Most of the cost estimates were in the form of engineering estimates prepared by reputable and qualified U.S. engineering and construction firms. A few were in the form of budget estimates which, of course, are subject to some variation when final costs for construction are prepared. One such instance occurred while this report was in preparation. For budget purposes, a company included in this study prepared an estimate of $50 million at the time of the Bureau’s examination. The engineering company estimate for design modification and construction of the facilities was $75 million. Firm bids for construction will be considerably higher than a number of the estimates included in these capital cost summaries.
The cost estimates studied in the Bureau’s survey have not taken any credit for sale of sulfuric acid or other sulfur byproducts or cost for their disposal. Some smelters are so situated that they will be able to dispose of their acid production for some positive value, and thereby reduce the effective cost of environmental controls. Other smelters, less favorably situated, either will have to subsidize transportation charges to deliver the acid to a market or will have to neutralize it at the smelter site. To these smelters sulfuric acid will be a byproduct debit.
The estimates did not include an allowance for lost production during the period of constructing the control facilities nor for the costs incurred during periods of shutdown for maintenance after the facilities are operating. Based on the detailed examination of the company estimates, the Bureau of Mines estimates that the figures are reasonably accurate, probably within plus or minus 15 percent. It appeared to the Bureau engineers who made the examination that all of the corporate capital cost estimates were for the purpose of sulfur dioxide control. There were no clearly identifiable costs that could be attributed to increased capacity or economy of production. On the other hand, it was evident that the cost of operating the existing plants, in conjunction with the new control facilities, would add substantially to the cost of smelting copper. Assuming that it might be possible in a detailed review of the capacity of unit operations to determine that certain portions of the facilities would provide some additional capacity over that of the existing plants, it is likely that the percent of capital which could be so identified would be within the accuracy of the capital cost estimates.
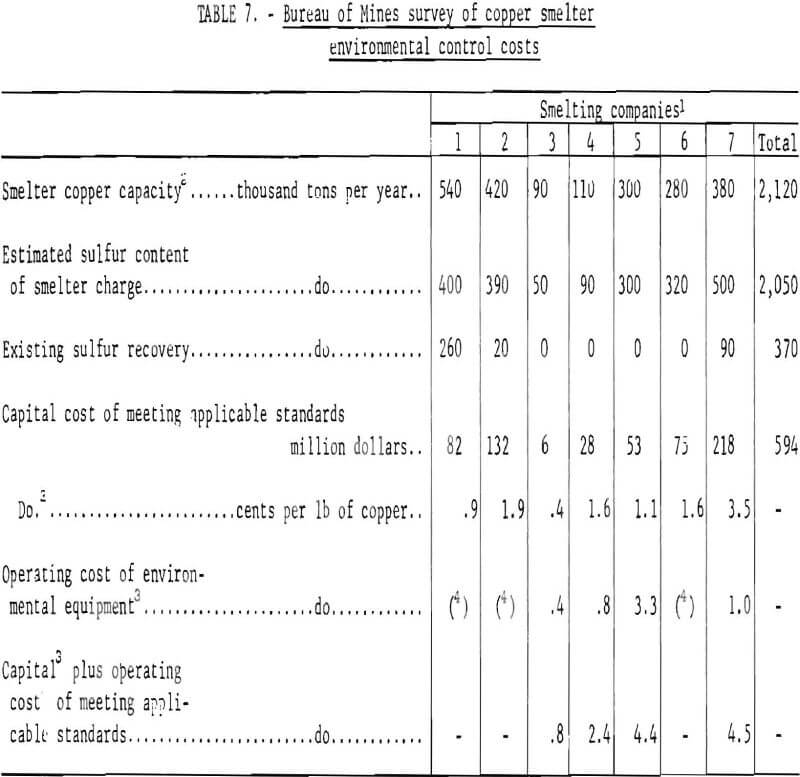
A total cost estimate has not been compiled for the lead arid zinc smelting industries, however, from the company cost estimates that were examined by the Bureau. The capital cost is expected to exceed $100 million and the production cost will probably increase by 2 to 4 cents per pound for lead and 1.5 cents per pound for zinc. A comprehensive study of the lead and zinc smelting industries will have to be made to confirm the accuracy of these cost estimates.
Regardless of the exact amount of capital expenditure required, outlay of huge amounts of capital demands careful planning to assure that the control methods selected will be effective and reliable without transforming the air pollution problem into one of land or water degradation. In addition, a solution that minimizes adverse international trade implications will be reached only if there is tolerance in the time allotted for reducing emissions, particularly for those smelters that cannot readily dispose of sulfuric acid. In the end, the public interests should be best served if allowances are made for the difficulty in bringing technology to a working practice.
