Microwave Acid Dissolution of Metal Samples
Objective
Reduce or prevent acid mine drainage from coal refuse piles and surface mines by inhibiting the growth of acid- causing bacteria.
Approach
A dilute surfactant or detergent solution is applied directly to coal refuse piles or overburden using a hydroseeder or road watering truck. The surfactant treatment can be used either as a preventive measure to avoid a potential acid drainage problem or to reduce water treatment costs by controlling acid drainage at its source.
How It Works
Acid drainage is prevented or reduced by inhibiting the growth of Thiobacillus ferrooxidans, a type of bacteria which obtains most of the energy it needs to survive by oxidizing ferrous iron in water, The oxidized iron in turn attacks pyrite, which forms an acid and additional ferrous iron for the bacteria to oxidize. T. ferrooxidans is protected by an outer membrane which enables it to survive in its acid environment. Anionic surfactants, or surfactants containing negatively charged ions, can be used to destroy this membrane, thus killing the bacteria and slowing down the oxidation of acid-forming pyrite.
Of the anionic surfactants tested to date, sodium lauryl sulfate (SLS) appears to be the most effective as a bactericide. Alpha olefin sulfonate and alkyl benzene sulfonate are acceptable alternatives. These surfactants are major components of common household products, such as laundry detergent, shampoo and toothpaste. They are, therefore, readily available, inexpensive, biodegradable and present no environmental problems at low concentrations.
The amount of surfactant required for a given application varies with the site being treated. Adsorption in rock and soil limits percolation of the surfactant through the pyritic material. Therefore, more surfactant should be used to compensate for that which is adsorbed. However, the adsorbed surfactant does apparently slow down repopulation of the bacteria.
To determine how much surfactant should be applied, conduct this simple test to determine the adsorptive capacity of the material to be treated. Pack the material into a perforated vessel and
treat it with 50 ml of a 500 ppm surfactant solution. Place a second perforated vessel on top and connect this vessel to a rinse solution reservoir consisting of either distilled water or dilute surfactant solution. Collect the rinses and analyze them to determine the amount of detergent retained by the material.
A typical coal refuse adsorbs about 0.05 mg of surfactant per gram of refuse. This corresponds to an application rate of about 55 gallons of 30 pct SLS concentrate diluted to about 50 ppm in order to satisfy the adsorptive capacity of an acre foot of the refuse. In a refuse pile, where oxidation is generally limited to a near-surface layer, this application rate is appropriate. Greater penetration is needed for typical pyritic overburden material but adsorption is generally lower, so that application rates typically range from 20-60 gallons of 30 pct solution per acre. If non-pyritic material overlies the acid-forming overburden or refuse, surface application is still possible, but enough surfactant should be administered to compensate for the adsorptive capacity of all the material. This administration technique should not be used where a clay blanket exists or where compaction severely limits permeability.
Test Results
Field tests have been conducted on a ten-acre, inactive coal refuse pile near Beckley, West Virginia, and on an active section of a refuse pile near Kingwood, West Virginia. The quality of the drainage from the inactive site has been monitored for over a decade, providing a good baseline against which the effectiveness of the method could be judged. At the active site, a channel was constructed to collect surface runoff from the treated area so that water quality could be monitored. Both the inactive and active sites were treated by a hydroseeder which administered SLS at an application rate of 55 gallons of 30 pct solution per acre. At the inactive site, the SLS was diluted approximately 500:1; at the active area, a shortage of good quality water resulted in a dilution ratio of only 50:1. Water samples were collected at least once a week and routinely analyzed for sulfate, acidity, pH, Iron, and anionic surfactants.
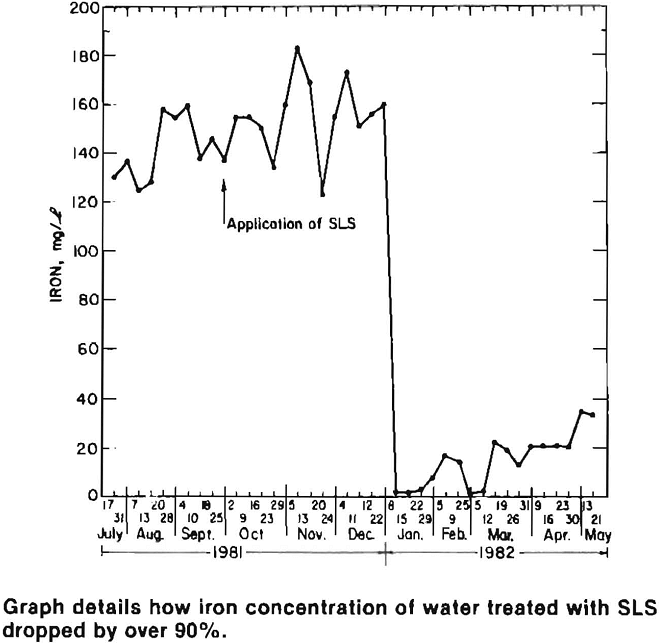
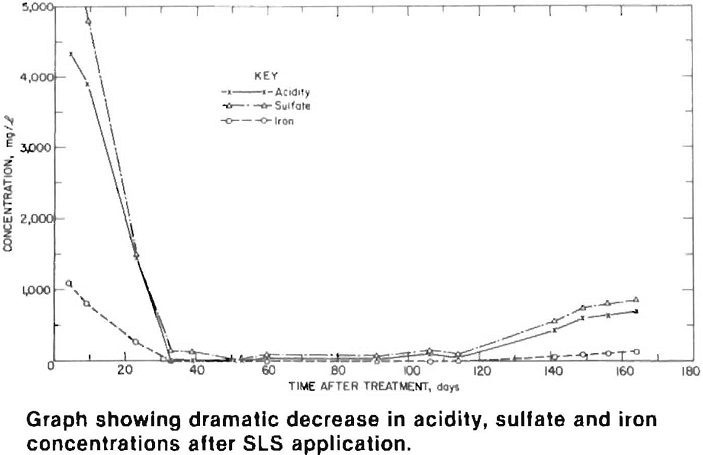
Water quality at the active site improved dramatically within a month of the SLS application. Acidity, sulfate and iron concentrations were reduced by more than 95 pct and remained low for four months after treatment. At the inactive sites, water quality improved after a three month lag period, which was primarily caused by stored acidity (sulfate salts) and the time required for surfactant infiltration and for it to flow through the pile and emerge as drainage. Acidity, sulfate and manganese levels decreased 60 pct, while iron concentrations dropped by over 90 pct.
Additional field tests are underway on surface mines and refuse areas in Pennsylvania, Ohio and West Virginia. At all sites, drainage concentrations of SLS have remained very low (0.20 mg/l or less) due to adsorption, SLS chemical decomposition, dilution and biodegradation. To avoid potential problems of surface runoff, the SLS should be applied during dry weather.
