The object of this article is to point out the experimental relationship which exists among contact angle, adsorption density, zeta potential, and flotation rate data. In each of the experiments discussed in this article, the surface properties of quartz, as a function of pH, were measured in solutions to which constant quantities of dodecylammonium acetate were added. The pH of the solutions was regulated with HCl or NaOH in all cases. In each case, the addition of collector was constant, but the concentration of dodecylammonium ions decreased because of the formation of dodecylamine at higher pH values.2
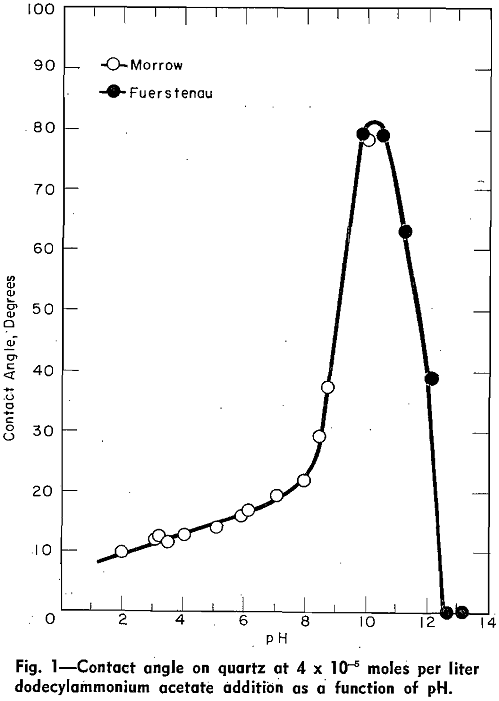
Contact Angles: Gaudin and Morrow presented data for the contact angle of quartz in solutions containing 4.08×10 -5 moles of dodecylammonium acetate per liter between pH 2 and pH 10.15.
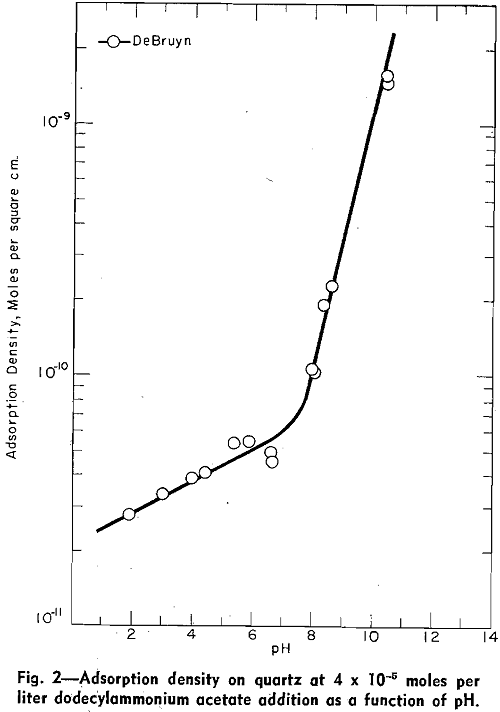
Absorption Density: Using carbon-14 tracer techniques, deBruyn measured the adsorption density of dodecylammonium ions on quartz as a function of pH in solutions containing 4.08×10 -5 moles of dodecylammonium acetate per liter.
Zeta Potential: Using streaming potential techniques, the writer measured the zeta potential of quartz in solutions containing an addition of 4×10 -5 moles of dodecylammonium acetate per liter as a function of pH.
Flotation Rate: Seele studied the rate of flotation of quartz using the modified Hallimond flotation tube. In this cell 1.80±0.04g of 48 to 65-mesh quartz was floated at the time necessary for recovery of about 85 pct under the optimum flotation conditions. This turned out to be 10-sec flotation at pH 10 with an addition of 4×10 -5 moles of dodecylammonium acetate per liter.
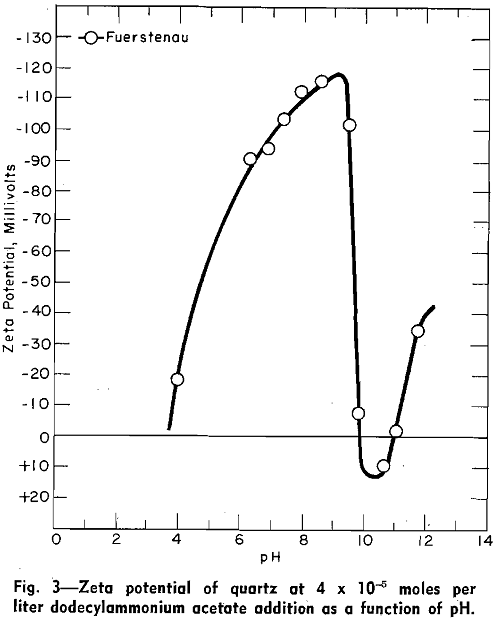
Increasing the alkalinity of the solutions with NaOH has a three-fold effect on the system: (1) the negative charge at the quartz surface is increased (2) the concentration of dodecylammonium ions in solution is decreased through the formation of dodecylamine molecules; and (3) the concentration of sodium ions is increased.

