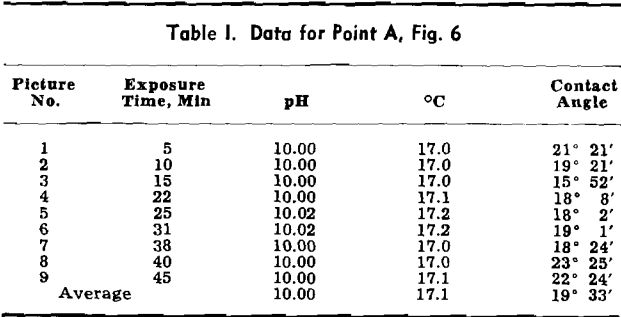
In the use of free bubbles with precise temperature control and continuous pH measurement, the contact angle apparatus differs from all previous equipment. Experimental procedures differ sharply from the captive bubble method of introducing the gaseous phase in the three-phase system. When freed from the tube and captured by a solid surface, a bubble more closely conforms to the spherical ideal set up by the usual mathematical analysis of surface free energy or surface tensional relationships of the forces involved.
The apparatus allows testing over a broad range of closely controlled conditions, and the use of free bubbles gives contact angles independent of operator manipulation, which was unavoidable in the older captive bubble method. A bubble released below the specimen rises through the liquid and contacts the mineral.
An electrode support groups the four electrodes so that they fit conveniently into the reaction cell. This assembly holds the glass electrode, the calomel electrode, the temperature-
compensating thermocouple for the pH system, and the thermocouple for temperature indication. The temperature-indicating thermocouple is glass-insulated 24-gage iron-constantan which is cemented in pyrex glass tubing. The exposed spot-welded junction of the thermocouple is given a light plastic coating to prevent corrosion of the wire. The thermocouple is connected to the electronic temperature indicator, which has a temperature range from 0° to 100°C and 0.2°C scale subdivisions. Leads from the three electrodes for the pH system go to a Beckman Model R indicating amplifier, which has a range from pH 3 to 10.
An optical bench is used to support the light source, the condensing lens, the thermal jacket, and the microscope, eyepiece-viewer, and 35-mm camera. Suitable mounts on standard carriages have been designed to hold each piece of apparatus, permitting easy optical alignment. There is no operational time limit on illumination, which is provided by a high intensity mercury lamp with a steady arc requiring no adjustment.
Experimental Procedure: A solution containing a surface active agent is placed in the reaction cell, which has a volume slightly in excess of 500 ml. The reaction cell is then transferred to the thermal jacket, O-rings are inserted into the recessed grooves of the optical ports, and the ports are tightened against the cell walls. After standardizing with an appropriate pH buffer, the rinsed electrodes are positioned in the reaction cell. Time required for the circulating system to bring the test solution to temperature depends on a predetermined setting for this environmental factor. Once the system comes to the desired temperature, it may be maintained within ±0.2°C.
A stream of distilled water and a cloth-covered glass lap are used to buff the previously polished specimen on a variable speed polishing wheel. Experimentation will determine the type of polishing cloth and the abrasive to be used on various minerals.
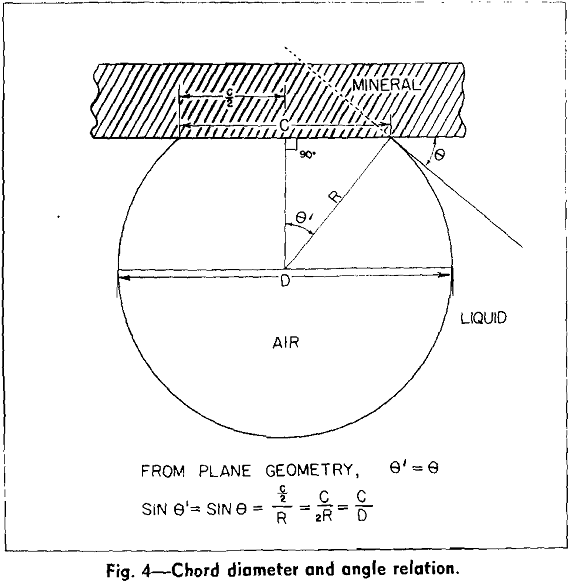
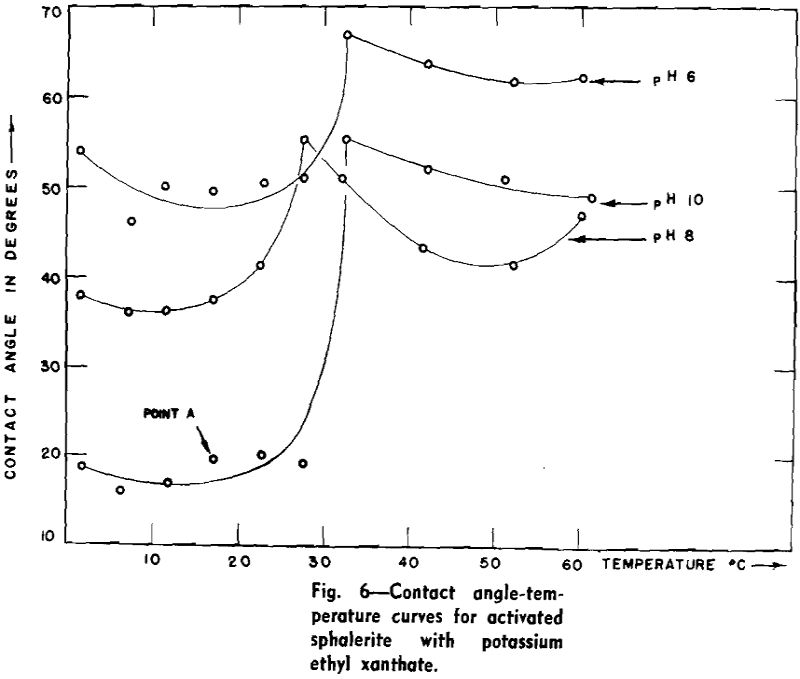
Bubbles captured under equilibrium conditions always have a finite contact angle. The magnitude of the angle is determined from the photographic negative by measurement of the chord and the diameter of the segmented spherical bubble.
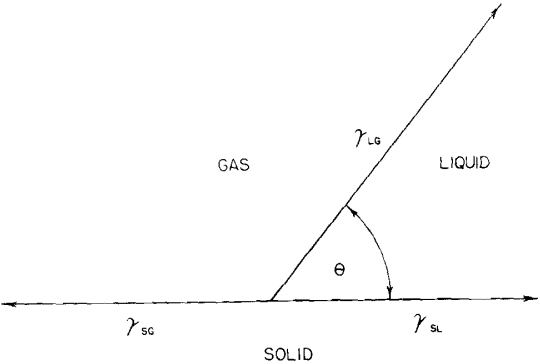
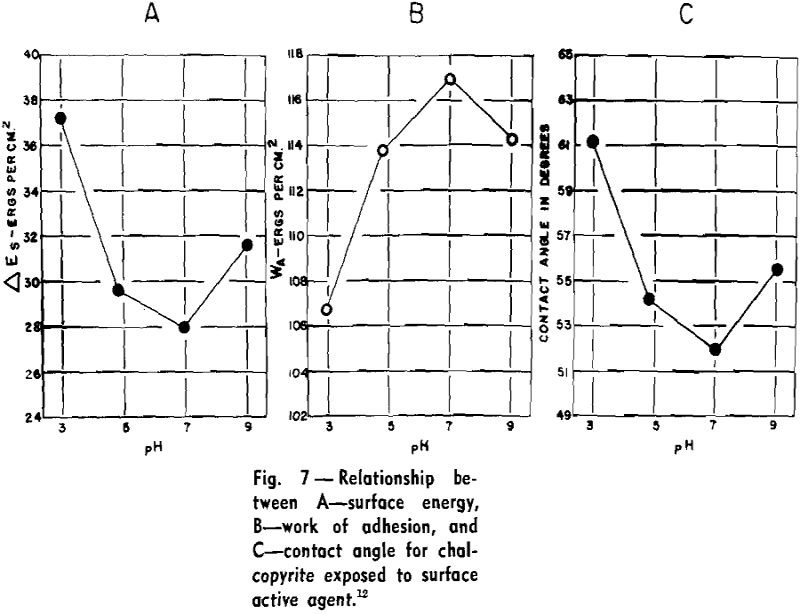
Theoretical Considerations: Contact angle is a definite and real physical quantity characteristic of a three-phase system. Conventionally, it is measured within the liquid, and it is the angle between the liquid-solid surface and the liquid-gas surface. Dupre first formulated the relationships between the surface energies or surface tensions to obtain the adhesional work involved in the approach of two unlike surfaces. The work of adhesion (Wα) makes it possible to express the energy difference in the three-phase system as Eq. 1:
![]()
The decrease in surface free energy (ΔEs) of the interfacial system has more thermodynamic significance than work of adhesion. ΔEs is also a function of θ, the contact angle and γlg, the surface tension of the liquid-gas interface. When surfaces of unit area are compared:
ΔEs = -γlg (1 — cos θ)………………………………………..[2]