Table of Contents
Agglomeration, as used throughout this report, refers to a mineral dressing process in which the desired mineral, namely cassiterite, is selectively separated from the gangue minerals by attachment to a crude oil. This process is analogous to flotation except that the sought after mineral is collected by crude oil rather than air bubbles. The ore is ground and conditioned to make the valuable mineral, or minerals, oleophilic and then they are collected, or agglomerated, by the crude oil. The mechanical action involved in this process is one of mixing solids and oil so that the conditioned solids will adhere to the oil when brought into contact with it. The several methods of mechanical mixing used in this process to date will be discussed below.
Experimental Work
The experimental work in this investigation has taken two general directions, one to examine the chemical aspects of the process, the other to examine the mechanical aspects. The mechanical aspects of the process included apparatus design, method of contacting oil and solids, comminution of the ore, duration of contact, method of collection and feed rates. In examining the chemical aspects of the process, the effect of pH, various conditioners and collectors were examined.
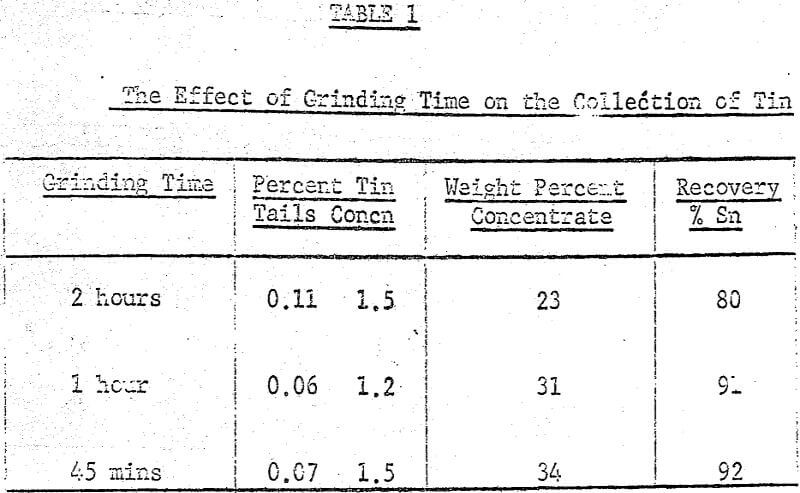
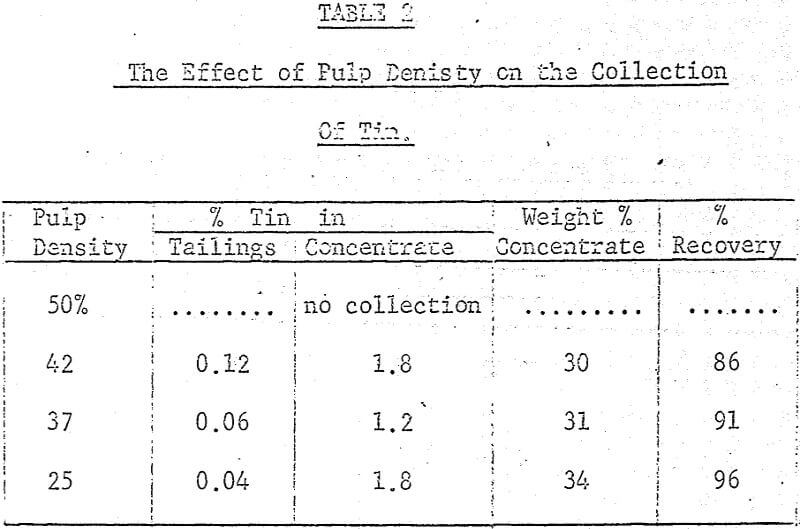
After several preliminary experiments a set of standard operating conditions were established and from them a systematic examination of several variables, both chemical and mechanical, were carried out. The standard procedure adopted was to place 100 grams of ore (-8 mesh) in the ball mill with the flint or porcelain charge along with 200 grams of water, 0.2% (based on dry weight of solids) crude tall oil and 3% Lloydminster crude oil. This mixture was then placed on the rollers for two hours and grinding and agglomeration took place simultaneously.
Substances examined as possible conditioners included soluble salts of fatty alcohol-sulphates, petroleum sulphonates, napthenic acids, fatty acids and their derivative, arsonic acids, oxidized and sulphonated hydrocarbon oils, fish oils, inedible tallow, non-ionic compounds of fatty acids and thiourea. With minor variations these materials all gave good recoveries of tin and the sulphide minerals. Derivatives of arsonic acid gave somewhat improved selectivity for tin and therefore improved the grades. Other polar derivatives of hydrocarbons such as alcohols, ketones and phenols did not show sufficient activity to be attractive.
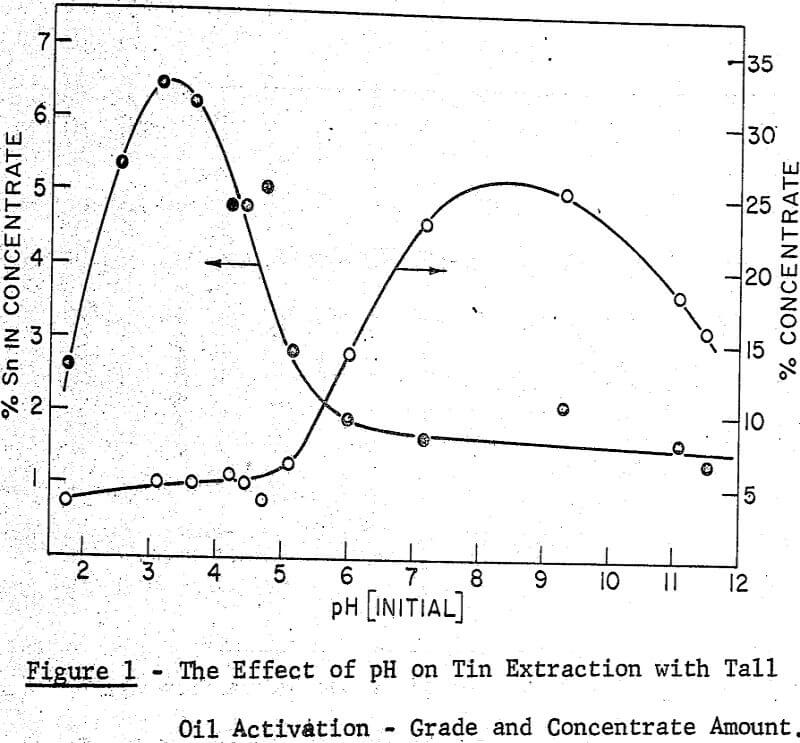
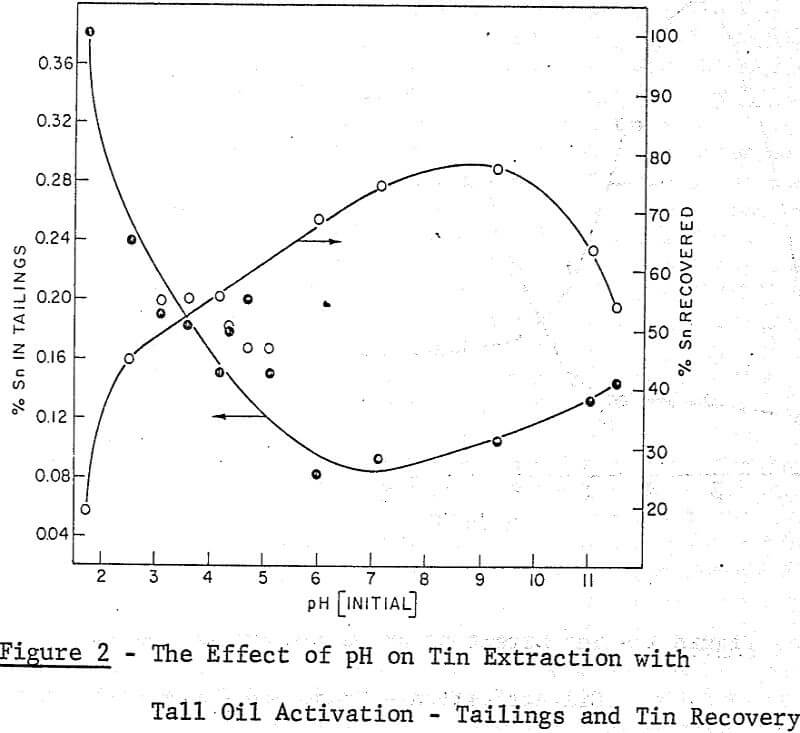
A maximum tin grade in the concentrate of about 7 percent, and a minimum in the tailings of about 0.08% were obtained, but unfortunately not at the same pH values, hence any one-step operation would therefore seem to require a compromise between grade and recovery. The pH values recorded in the graphs were taken after 5 minutes of grinding and they were adjusted with either sulphuric acid or sodium hydroxide.
A typical experiment was to grind 500 grams of Mount Pleasant ore (designated MTL 6) in 350 cc of water, with 0.2% tall oil, in a 6 inch interior diameter porcelain mill with 75 three-quarter inch steel balls for one hour. The minus 20 mesh fraction of this material was then pulped with more water to give a mixture containing 30% solids. This mixture was then fed to the grease kettle beaker in lots of approximately 700 cc to be agglomerated with 150 grams of oxidized Lloydminster crude oil which had been placed in the grease kettle. The stirring action was allowed to continue for 15 minutes and the kettle was flushed out with water which entered through the bottom and over-spilled via the trough at the top taking with it uncollected oleophobic material.
Continuous Agglomeration
In order to agglomerate a tin ore continuously a new piece of apparatus was constructed. The agglomerator consists of a 2 inch interior diameter glass tube 42 inches long with glass T’s at both ends. A stirring device, duplicating the stirring action of the grease kettle, was contained inside the glass tube. Two sets of paddles rotated in opposite directions, as in the grease kettle. The shorter, interior set was twisted so that they served to push the agglomerate through the tube like a screw conveyor. The outer set of stirrers served as a scraper to clean off the inner wall of the tube and to knead the agglomerate. Conditioned pulp and oil could be admitted to one end of the tube, passed through the stirrers and ejected at the other end of the tube.
Several experiments were carried out in which the oil rate, that is the amount of oil added to the agglomerator per unit time, was altered. The oil was varied from 2.6 grams per minute to 0.6 grams a minute and both oxidized and unoxidized oils were used. Results showed that with an oxidized crude oil rate of 1.5 grams per minute a recovery of 97% with a grade of 1.0% Sn could be obtained, while with an unoxidized crude oil rate of 0.6 grams per minute a recovery of 93% and a grade of 1.9% Sn was obtained.
The feed material, in all experiments except one, was minus 20 mesh. In one experiment minus 100 mesh feed was pulped to 15% solids and agglomerated with unoxidized crude oil. This material gave a recovery of 81% with a grade of 1.9% Sn and a small (20%) amount of material in the concentrate.
In a test in which the concentrate was milled with water and HCl, a high rejection of material of low Sn value was obtained. In another test using phosphoric acid with water, the tails or reject portion was high in tin (2.5%). A series of experiments was therefore set up, in which a concentrate from the agglomerator, containing about 1.5% Sn was milled with water, the tails removed, then milled with water and HCl (pH=2.0), the tailings again removed, and then milled with H3PO4. This experiment was repeated several times using Mount Pleasant concentrates and once each with Cornish Tin Tails concentrate and Cominco Blanket Feed concentrates, and in all cases an enrichment of the tailing portion was noted after the H3PO4 treatment. Table 5 shows the results obtained, with Cornish Tin concentrates.
It may be noted that, throughout this work the experimental conditions which gave the best performance in the ball mill work were also effective in the grease kettle and the continuous agglomeration tests. From the pH effect reported in the Farnand, Meadus, et al publication it may be said that an optimum recovery of tin occurs between a pH of 8 and 9. This was also noted in the grease kettle work where a pH of 8.6 gave the best results.