Table of Contents
Blast furnace smelting of lead began over 100 years ago. The preparation of feed for the smelting operation always has been an important factor especially the elimination of sulfur by roasting. Over the years roasting progressed from heap roasting to reverberatory roasting, blast roasting in pots, and finally to continuous blast roasting of thin layers by sinter machines. In some of the earlier roasting systems lead was produced by the roast reaction process and separated from the charge. The gray slag material from the roast reaction process and high-silica ores served as feed for the lead blast furnace. Lead sintering, as used in the lead industry today, is a method for preparing feed for the lead blast furnace. It serves two purposes; first, it is a method for removing sulfur from the concentrate, and second, it is a method for agglomerating the lead concentrate into a product suitable for the blast furnace.
In normal sintering operations for preparing feed for the lead blast furnace, lead concentrate is mixed with slag, return sinter, and fluxing agents and the mixture is pelletized and sintered. The mixture consists of approximately 25 pct high-grade lead concentrate, 50 pct return sinter, 15 pct slag, and 10 pct fluxing agents, dusts, etc. The sintered product is crushed and sized with the undersize material being recycled to the sinter machine and the oversize serving as feed for the blast furnace. The finished sinter contains approximately 50 pct lead.
In sintering to produce a 70 to 80 pct lead feed for the self-fluxing furnace as described by Schwartz and Haase and G. Pungartnik and others, no fluxes or slags are added to the sinter charge. The charge consists essentially of 20 to 25 pct high-grade concentrate, 70 to 80 pct return fines, and 2 to 4 pct flue dust. The sintered product is screened to produce a coarse sinter hearth layer, a fine fraction to add to the next sinter charge, and a finished sinter containing 73 to 83 pct lead and approximately 4 pct sulfur. This product, although not acceptable as blast furnace feed, is fed to the short rotary furnace where the roast-reaction process produces lead. The slag formed by the small amount of contained silica is treated separately with soda ash and carbon for recovery of the lead.
Wallden, Lindvall, and Gorling developed a method for sinter roasting of lead-rich concentrates without slag or silica additions to produce a high-lead product for electrothermic roast – reaction-smelting. This method utilizes a sandwich pellet built up around an 8- to 20-mm core of recycled sinter. A three-stage pelletizing operation is used to build up concentric layers of lead oxide dust, lime, and concentrate. The layers around the central minus 20- plus 8-mm core range from 1 to 2 mm in thickness. The sintered product is crushed to 30 mm (approximately 1 inch), screened on 20-mm and 8-mm screens. The plus 30-mm material serves as a hearth layer for the sinter machine, the minus 20- plus 8-mm portion goes to make up the core for new pellets and the minus 8-mm (2-mesh) fraction goes to the electrothermic furnace. The amount of slag produced in the rotary furnace is relatively small compared with standard lead blast furnace technology and is allowed to build up to be periodically treated with soda ash and carbon for recovery of the lead. The finished sinter contains about 70 pct lead and 6 pct sulfur (4 pct sulfur as sulfide and 2 pct sulfur as sulfate). The sinter would not be suitable for use as blast furnace feed.
Many basic studies have been made relative to PbS roasting and Pb-S-O equilibrium reactions and the variety of lead-sulfur oxygen compounds formed under the various roasting conditions have been reported. Kellogg and Basu reported on the reactions during the oxidation of PbS at temperatures up to 827° C. Lloyd extended the study from 827° to 1,052° C. Both of these investigations measured the pressure of SO2 gas in equilibrium with the condensed phase.
Schenck and Albers and Kirkwood and Nutting discussed the chemical equilibrium between lead sulfide and its roast products. Wittung quantitatively described the equilibrium reactions which occur in the roasting process under various conditions of temperature and oxygen concentration. The temperature range studied varied from 627° to 877° C, and oxygen content, from 0 to 20 mole-pct. The relationship between lead, lead sulfide, lead sulfate, and basic lead sulfates was pointed out.
Culver, Gray, and Spooner controlled the composition of roasting gases to study the kinetics of the reactions which formed PbSO4 and PbO·PbSO4 between the temperature range 673° to 807° C.
Tuffley and Russell calculated the stability regions of the normal sulfate and basic sulfate of lead in O2-SO2 and PbS-SO2 gas atmospheres over the temperature range 600° to 1,200° C. They applied their results to the sintering of lead concentrates to determine the extent to which each of the various reactions occurred and pointed out that sulfates are formed only in minor amounts during actual sintering because of the rapid removal of SO2 from the reaction zone. Lead sulfates which tend to form were subjected to reduction by PbS vapors.
Kohlmeyer and Monzer pointed out that PbS and PbO appear to form a eutectic system with each other when heated quickly up to 900° C and the system decomposes at temperatures above 950° C to form Pb and SO2. Yazawa and Gubcova in presenting the results of thermodynamical calculations which were made on the Pb-S-O system at 680°, 900°, and 1,100° C indicated that the roast reaction process to produce lead can proceed very readily. Kirkwood and Nutting investigated the oxidation of galena crystals in air below 200° and 350° C and determined that PbSO4 is the first product to form followed by PbO·PbSO4 and 4PbO·PbSO4.
Studies on lead sulfide roasting confirm that the roast reduction process should be an ideal method for recovering lead from lead sulfide. However, Penko has stated that it is practically impossible to provide satisfactory control over the reactions between gaseous and solid phases when roasting sulfides in multihearth and drum-type furnaces because of the impossibility of measuring or controlling temperature. Schwartz and Haase further pointed out that much of the reported work on the roasting of lead sulfide was done on lead compounds in the solid state but that in actual operations the molten state and combinations of molten or semimolten material was common-place. They also recognized that the reaction of PbS upon PbO would be impeded in actual furnace operation where ores contain silica since lead silicates would be formed which cannot be easily decomposed by PbS.
The effect of silica and other nonlead compounds on the roasting of lead sulfide has been largely ignored in the basic studies on Pb-S-O even through the affinity of lead oxide for silica has been widely recognized for many years. The formation and constitution of lead silicates was studied in the early 1900’s by Simmons, Manchot and Kieser Mostowitsch, Cooper, Shaw, and Loomis, Hilpert and Weiller, and Hilpert and Nachen, Hilpert and Nachen showed two chemical compounds, 2PbO·SiO2 and 3PbO·SiO2 and three eutectics, one of PbO-2PbO·SiO2, a second of 2PbO-3PbO·SiO2, and a third of 3PbO·2SiO2 -PbO·SiO2. Mostowitsch has shown mixtures of PbO and SiO2 ranging in composition from 6PbO·SiO2 to PbO.
In the mid-1930’s, Geller, Creamer, and Bunting studied the PbO·SiO2 system and identified three components, namely 4PbO·SiO2, 2PbO·SiO2, and PbO·SiO2. The melting point of the compounds was 725°, 743°, and 764° C, respectively. In Preston and Turner’s studies relating to the surface tension of PbO·SiO2 system, they indicated that the surface tension of lead silicate melts is increased with increased percentages of silica and that PbO volatility from the melts decreased with increases in silica content. From their study of the initial vaporization rates of PbO from the lead glasses, they indicated that PbO·SiO2, 2PbO·SiO2, and 4PbO·SiO2 were formed and that glasses containing 0 to 79 pct PbO were mixtures of SiO2 and PbO·SiO2; those 79 to 88 pct PbO were mixtures of PbO·SiO2 and 2PbO·SiO2; those 88 to 94 pct PbO were mixtures of 2PbO·SiO2 and 4PbO·SiO2; and those in excess of 94 pct PbO were mixtures of 4PbO·SiO2 and PbO.
The effect of silica on PbSO4 was recognized by Friedrich and by Borchers who indicated that PbSO4 was readily decomposed with silica at 950° C. Mostowitsch indicated that the decomposing effect of SiO2 and PbSO4 was governed by the viscosity of the lead silicate formed and that a viscous slag coating enveloping PbSO4 would retard the reaction of PbSO4 and SiO2. The adverse effect of silica on the roasting of lead ores was noted by Hofman who pointed out that ores containing more than 5 pct silica could not be treated by the roast-reaction process and that even as little as 0.5 pct silica makes itself felt by coating particles of ore with silicate.
Stehli was aware also of the PbO·SiO2 reactions in sintering when he pointed out that return sintering differed from double sintering since in double sintering PbO was not combined with SiO2 as in return sintering.
In a discussion of a paper by Beasley relating to high zinc in lead blast furnace slags, Arthur S. Dwight pointed out that there is likely to be a considerable formation of silicate of lead in the sintering of charge for the blast furnace. He pointed out that the lead may be largely in the form of silicate if sufficient silica is present, but in spite of this, blast furnace reduction would be almost complete and with less fuel than without sintering.
Although these facts have been known for many years, there is little indication in recent literature that lead silicate or complex lead-calcium- zinc -iron silicates are formed during sintering.
Hanson indicated that the major components of sinters were litharge and lead oxide compounds, metallic lead, spinels and ferrites, and melilites. He further indicated that the litharge grades off into a yellow-green material consisting of lead oxides and silicate compounds which form the bulk of the sinter bonds. It is not clear whether the silicate compounds Manson referred to were lead silicates or other silicates.
Isakov and Yanev reported on phase modification in a lead charge during sintering and indicated that lead sulfide was converted to PbSiO3 and PbO and that ferrite, melilites, calcium silicate, and willemite were formed during sintering.
O’Keefe, Bennett and Cole pointed out in this study of updraft sinters that the predominant lead phase present in sinter was a lead oxide silicate compound and that no lead oxide or basic lead sulfate compounds were identified except on outer oxidized edges of the sinter.
In reviewing the cited literature, the following list of compounds have been identified or concluded to be present in roasted PbS, lead oxide-silica melts, or in lead sinters:
Pb 2PbO·SiO2
PbS 4PbO·SiO2
PbO 6PbO·SiO2
PbO·PbSO4 Lead oxide glasses
2PbO·PbSO4 Ferrites (Fe, Zn)O·Fe2O3
4PbO·PbSO4 Melilites 2CaO (Zn, Fe)O·SiO2
PbO·SiO2
As a result of this review, various systems involving lead and other major constituents found in sinters were investigated in an effort to improve blast furnace feed. In so doing, the Rolla Metallurgy Research Center sought to produce an improved sinter product by analyzing the compositional phases of these systems and investigating the effect various constituents have on lead sintering. An improved sinter product should have a lead content substantially higher than present sinters, a low-sulfur content, and physical properties which would be compatible for use in existing blast furnaces.
Several of the sinter systems mentioned earlier produced sinters containing from 20 to 30 pct more lead than commercial blast furnace sinters but the resulting product from these sinter operations would not be acceptable blast furnace feed because of higher-sulfur content and lack of acceptable physical properties.
In our sinter studies we examined sinters produced by lead smelters, those prepared in sinter pot experiments and air-roasted mixtures of PbS, SiO2, ZnS, CaO, and Fe2O3.
This report summarizes the results of the microscopic and electron probe study on selected sinters and products and is in basic agreement with the conclusions of O’Keefe, Bennett, and Cole that lead oxide and lead sulfate play only a limited role in the composition of finished sinter.
Further, this report specifically points out that sintering is not a matter of controlled oxidation of lead sulfide but rather a complex set of interactions between all of the constituents.
Experimental Procedure
Samples of sinters produced on Dwight-Lloyd updraft type machine were obtained from several smelters operating in Missouri. Samples were taken at random from finished sinter prepared for the blast furnaces. Sinter samples were also obtained from laboratory sinter pot experiments. In addition, pure lead sulfide was mixed with varying proportions of silica, lime, zinc sulfide, and iron oxide and the mixtures were heated in air at 1,000° C for 1 hour. The products obtained by this treatment were compared with commercial sinter material,
Polished sections were made on several pieces of each of the sinters since apparent variations in the physical appearance of sinters were noted. Polished sections were also made on the oxidized mixtures of lead sulfide, silica, lime, zinc oxide, and iron oxide. Microscopic study of the polished sections indicated that numerous phases were present in all sinters. Phases were identified by optical microscope and by electron microprobe analyser methods.
A standard analytical technique was used for the microprobe analysis. The sample to be analyzed was first mounted and polished according to standard metallographic methods. The sample was not etched. High-purity elemental standards were used when possible, otherwise the standard was a well-analyzed compound standard. Both the standard and the unknown sample were carbon-coated to insure conductivity. An acceleration potential of 20 kV at 0.7 microampere beam current was used for all analyses. Elemental mapping was used to select the various phases with several points, usually four, per phase being analyzed. The regions being analyzed were generally 10 microns or more in diameter. Adequate count times were used to insure good statistics on all line counts. Background was determined on both the sample and standard for all points and lines by averaging counts above and below the line of interest.
The raw data were corrected for background, backscatter, ionization-penetration, absorption, and fluorescence by Colby’s MAGIC IV Computer Program for Quantitative Electron Microprobe Analysis.
Results of the microprobe analyses are the mean elemental composition and two sigma limits based on four analyses unless otherwise specified.
Lead Sinters
Lead sinter samples were obtained from the Buick smelter of AMAX of Missouri, the ASARCO smelter at Glover, Mo., and the St. Joe Minerals Corp. Herculaneum smelter. The sinters from the various smelters are similar in general composition although variations in relative concentration, morphology, and physical appearance do occur between individual samples whether taken from a single smelter or from the various smelters. Micrographs of typical sections of sinters are shown in figures 1 and 2. Microprobe analyses for individual constituents shown in figure 1A are given in table 1.
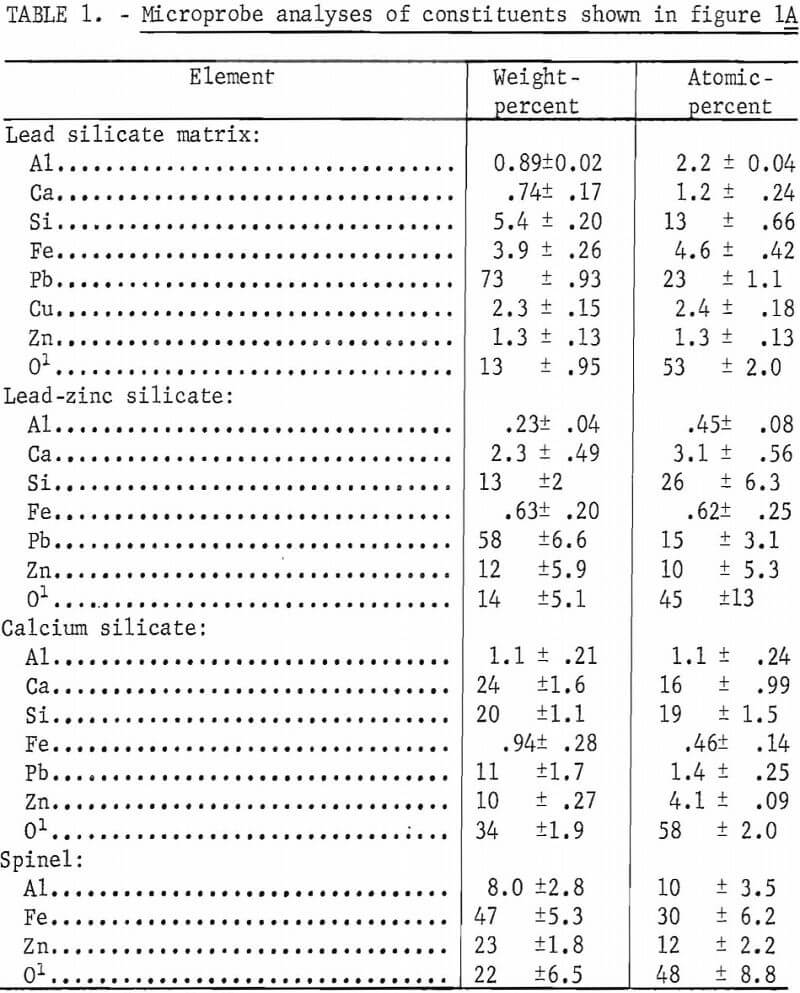
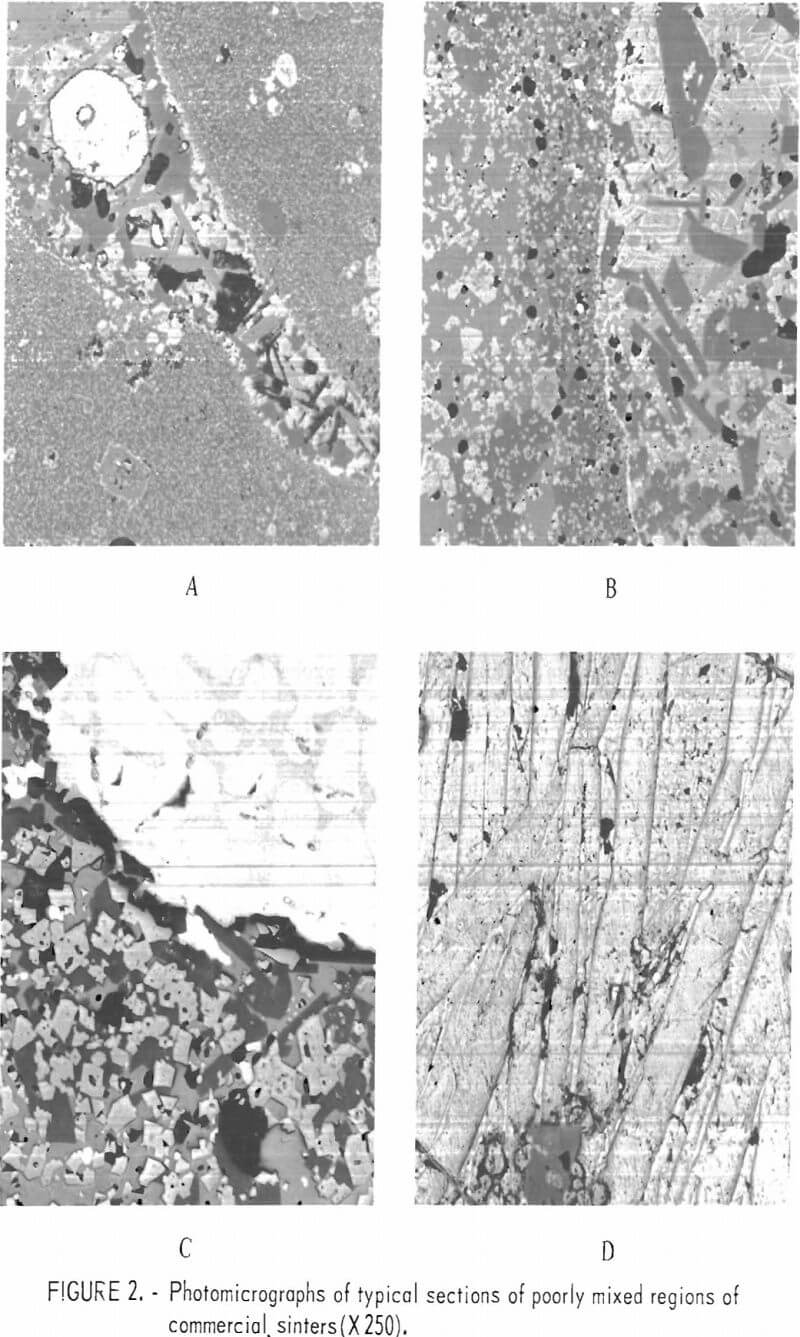
In these studies the existence of a zinc-bearing lead silicate was identified as a constituent associated with the lead silicate matrix. The zinc-bearing silicate appeared as light-gray angular grains within the lead silicate matrix. From microprobe analyses this component has a composition approaching (Pb, Ca, Zn)SiO2. The matrix phase more nearly approaches a composition of 2PbO·SiO2 with contained iron, copper, calcium, and zinc. These two phases, we believe, make up the B-1 phase reported by O’Keefe and others. The calcium silicate phase which appears as dark, blocky grains in figure 1A contains both zinc and lead and may be a zinc-lead-bearing wollastonite rather than a zinc-bearing melilite. This is the phase referred to as the B-2 phase by O’Keefe. The white inclusions (O’Keefe’s A-2 phase) which are normally referred to as ferrites contain both zinc and aluminum and are probably a spinel with a composition of (Fe, Zn)O·(Fe, Al)2O3 rather than ferrite. Lead metal and lead sulfide are present and areas of lead sulfates and oxides occur only sparingly in sinters which are suitable for the blast furnace.
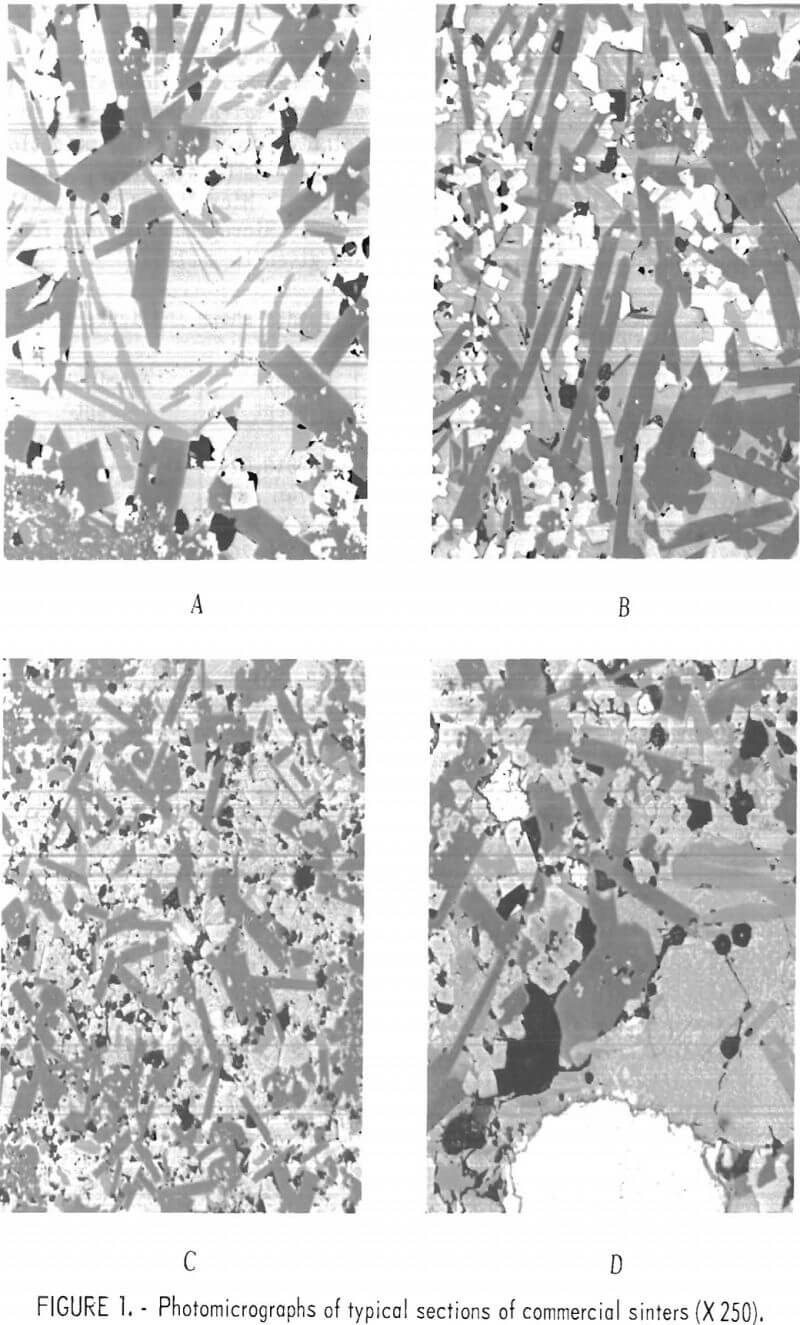
Figure 2 shows areas of sinter which do not represent the bulk of the sinter but which are an integral part of most sinters. These areas probably exist because of nonhomogeneity of the mixture or incomplete sinter reactions. Figure 2A shows an area of the typical crystalline silicate sinter as a wedge between two areas of fine-grained material which is virtually identical to lead blast furnace slag. This type of material was referred to by O’Keefe and others as the A-1 phase. These areas are probably unreacted, granulated slag particles which have been added to the sinter mix. Figure 2B shows one-half of the section composed of the fine-grained phase and one-half composed of the crystalline phase. Figure 2C consists of a large area of lead and lead sulfide surrounded by the crystalline silicate phase. The ferrite or spinel grains in this phase are abundant. Figure 2D is a phase seldom observed in the sinters that were studied. The large, lath-shaped crystals are composed of lead oxide and basic-lead sulfate. This type of structure has been duplicated in our studies on prepared mixtures by heating silica-free lead sulfide in air at 1,000° C.
Sintered Concentrate No Additives
A lead concentrate containing approximately 76 pct lead was pelletized and sintered with no return sinter, slag, or other additives. The analyses of the concentrate, in percent, are given as follows:
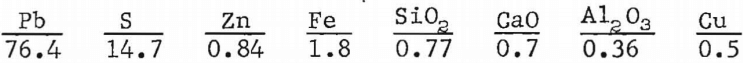
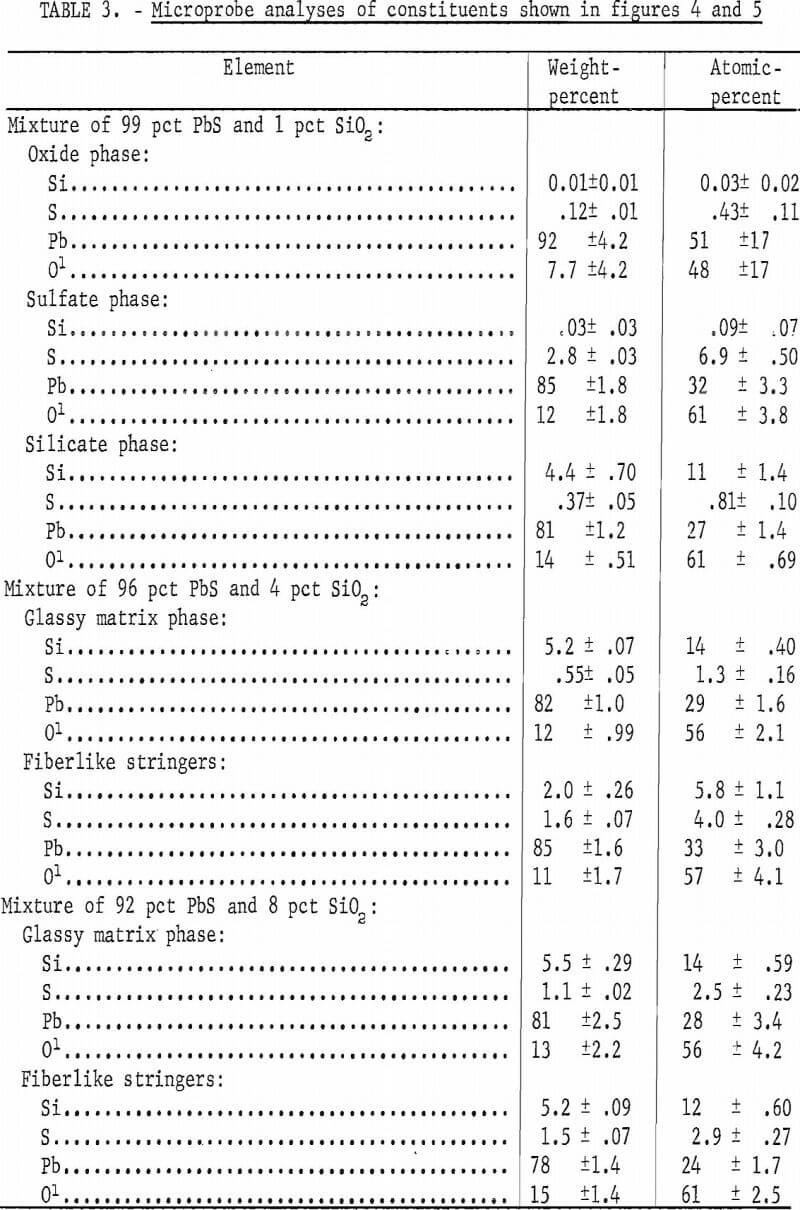
In attempting to sinter this concentrate, difficulty was encountered in achieving a uniform sinter. Areas were bypassed because of loss of sinter bed permeability, much lead was vaporized because of high bed temperatures and a very large amount of lead was produced by means of the PbS-PbO reaction. A micrograph of a typical region of sinter produced from high-grade concentrates is shown in figure 3. The constituents appear similar to those obtained in a commercially prepared sinter. Four constituents appear to predominate. These are a matrix phase, a dark-gray blocky phase, a light-gray angular phase, and a ferrite phase, Microprobe analyses of the phases are given in table 2.
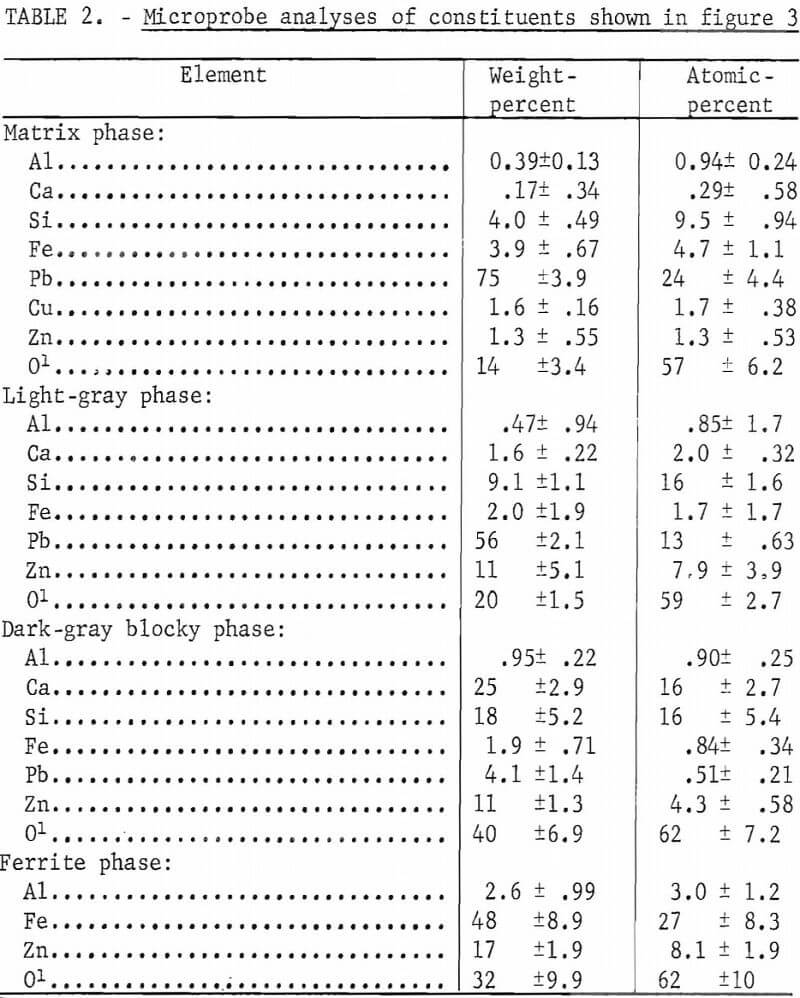

The matrix phase and the light-gray phase are lead silicates. The light-gray phase has an appreciable amount of zinc whereas the matrix phase has only a small amount of zinc but some iron. The dark-gray blocky phase is a zinc bearing calcium silicate and the ferrite phase contains both zinc and aluminum.
One of the important things to consider is that none of the products of the sinter shown in figure 3, other than the matrix phase, contains nearly as much lead as the original concentrate. The vaporization of lead oxide or sulfide and the reaction between lead oxide and lead sulfide has removed lead and simultaneously increased the percentage of zinc, iron, and silica in the remaining portion. The addition of silica to the concentrate before sintering undoubtedly has a pronounced effect on the losses of lead.
PbS-SiO2 Mixtures
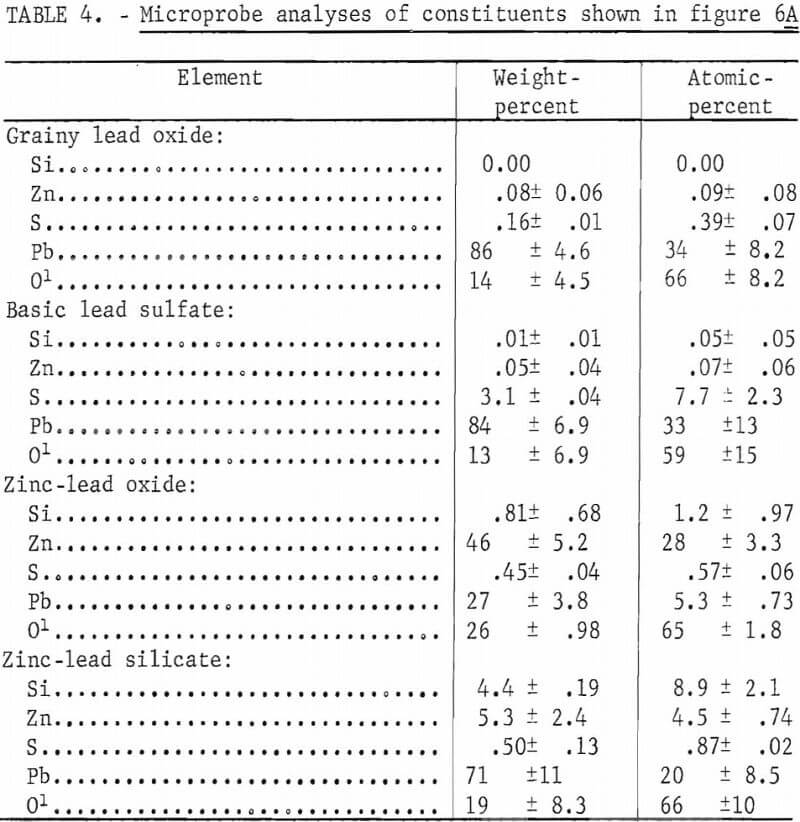
The effect of silica on the oxidation roasting of lead sulfide was considered important to the problem of sintering since most lead concentrates contain several percent or more of silica. Mixtures of PbS (hand-picked galena) and 1, 2, 4, 6, and 8 pct silica were prepared and oxidized at 1,000° C for 1 hour in a flowing stream of air. A small amount of lead was produced by the PbO-PbS reduction reaction. The sample was cooled and polished sections were made to examine and identify phases present. In addition, electron microprobe scans and analyses were made to aid in identifying the constituents. Photomicrographs of representative areas of the oxidized mixtures are shown in figures 4 and 5. The differences in the appearance of the roasted products are readily observable. The mixture containing 1 and 2 pct silica, respectively, are similar in appearance with each other and those containing 4, 6, and 8 pct silica are also similar to each other but much different from those containing 1 and 2 pct silica. The principal constituents of the 1- and 2-pct silica samples as determined by microscopic and electron microprobe analyses were lead oxide and lead sulfate with finely intermixed lead silicate. The oxide occurred as relatively large crystals and the intercrystalline material was intermixed lead sulfate and
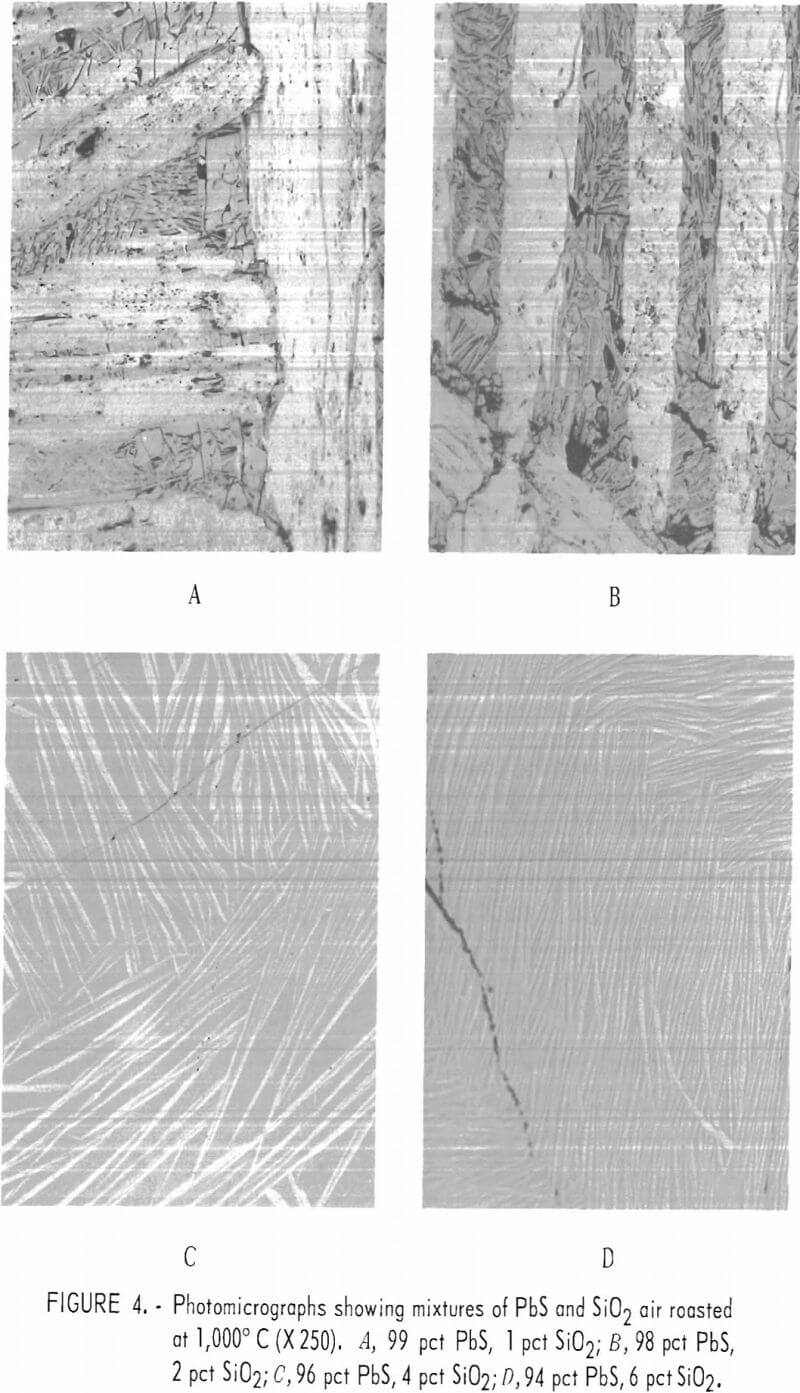

lead silicate. These sections were very similar to the section of sinter shown in figure 2C which we identified as lead oxide and basic lead sulfate. The 4-, 6-, and 8-pct-silica samples consist of a gray glassy matrix with elongated fiberlike stringers of a whitish material. Electron-probe analyses show sulfur to be present in both the glassy and the fiber like phases.
Electron probe analyses of areas of the various phases which occurred in the oxidized samples containing 1, 4, and 8 pct silica, respectively, are summarized in table 3.
The microprobe analyses of the PbS-SiO2 mixtures show that with only 1 pct SiO2 added the melt is composed of three phases; namely, a PbO phase, a basic sulfate (4PbO·PbSO4) phase, and a glassy silicate phase containing sulfur. With 4 or 8 pct silica mixed with the PbS the melts consist of a glassy phase and a fiberlike stringer phase. Both phases in these sections and the silicate phase of the 1-pct-silica sample contain sulfur and are probably composed of 2PbO·SiO2 with varying amounts of lead sulfate.
PbS-ZnS-SiO2 Mixtures
Addition of zinc sulfide (sphalerite) to lead sulfide-silica mixtures considerably alters the composition of the melts as reflected by the different phases shown in figure 6 as compared with figures 4 and 5 and by the microprobe analyses. Mixtures containing 1 and 2 pct silica, respectively, appear similar whether zinc is present or not. However, microprobe analyses show that zinc-oxide and zinc-lead-silicate areas are present in the sections represented by figures 6A and 6B. The mean elemental analyses for four components occurring in a melt from a 97-pct-PbS, 2-pct-ZnS, 1-pct SiO2 mixture represented by figure 6A are shown in table 4. These analyses show that the component, which appears as a grainy phase in figure 6A, is essentially lead oxide. The smooth appearing phase consists of basic lead sulfate, zinc-lead oxide, and zinc-lead silicate.
The change in the composition of the melt is more readily apparent in those melts with 6- and 8-pct-silica additions, respectively. With more silica the number and size of the needlelike inclusions and the amount of lead-sulfide dendrites forming in the glassy matrix is noticeable.
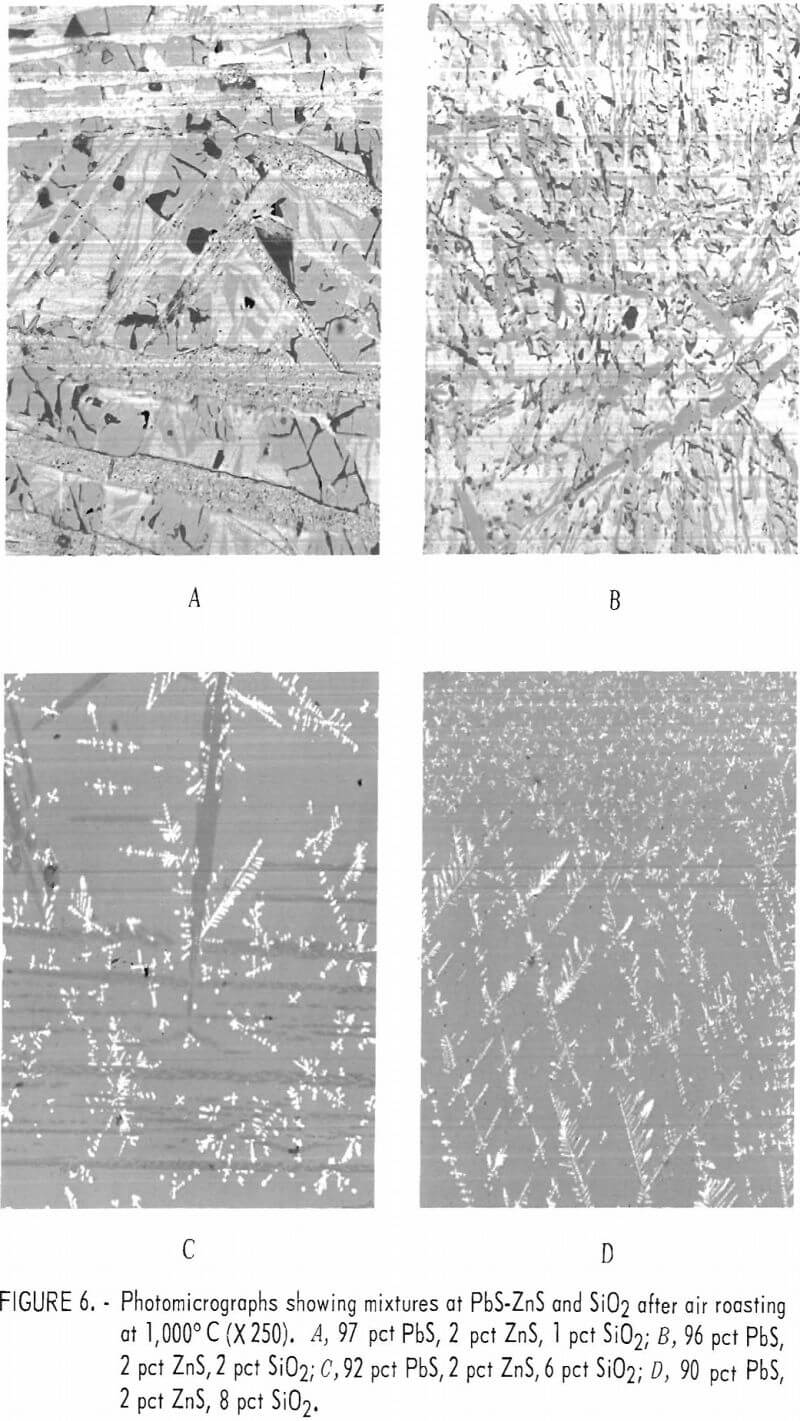
Microprobe analyses on the glassy and needlelike phases shown in figure 6c are given in table 5.
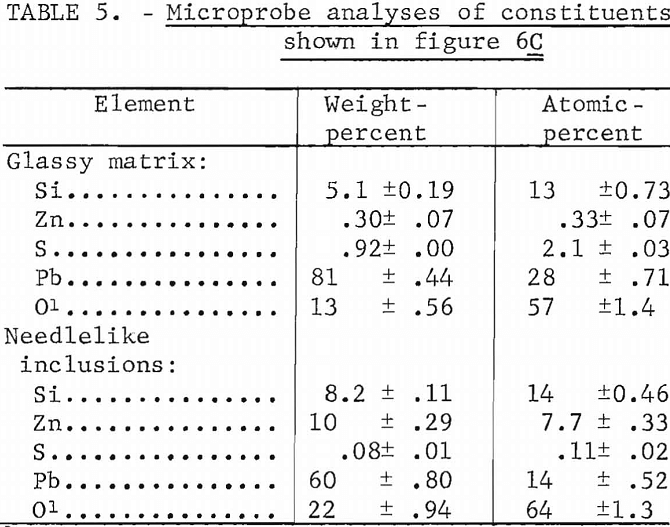
The matrix phase is essentially lead silicate containing a small amount of sulfur. The composition approaches 2PbO·SiO2. The inclusions within the
matrix have a composition approximating Pb2ZnSi2O7 which approaches the analyses by P. A. Van der Meulen of a silicate of lead and zinc from a lead-zinc furnace slag.
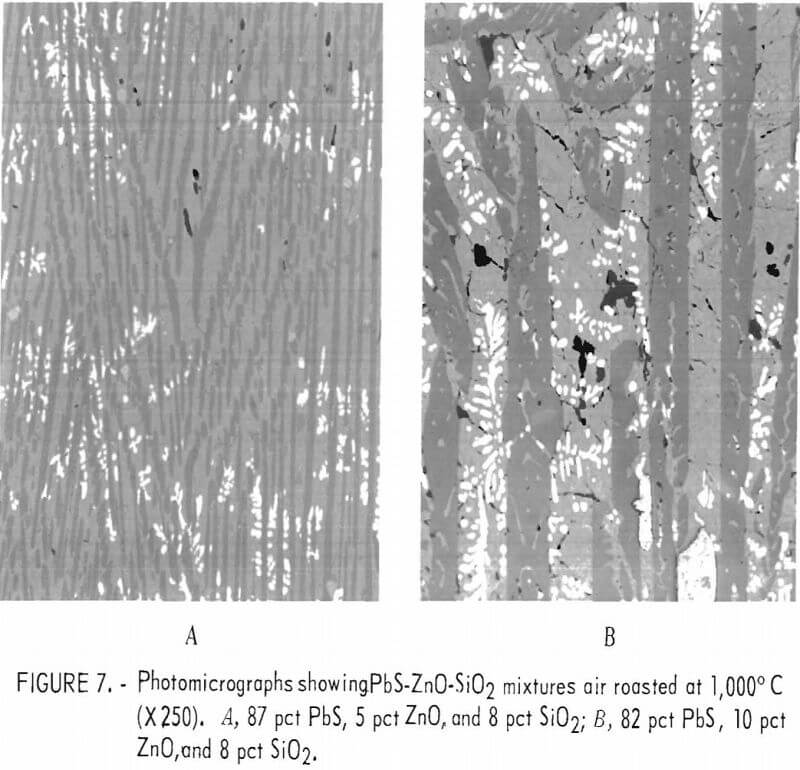
Increasing the amount of zinc in the mixtures from approximately 1.2 pct as shown in figure 6 to 4 and 8 pct by adding zinc oxide while maintaining the silica content at 8 pct resulted in an increase in the amount of lead-zinc-silicate formation as shown in figure 7. The presence of lead areas and lead-sulfide dendrites in the melts is readily seen. In the mixture containing 8 pct zinc (fig. 7B), a darker phase is shown within the dark lead-zinc-silicate phase.
The microprobe analyses of the silicate phases shown in figure 7B are given in table 6. The lead silicate phase is shown to contain appreciable sulfur and is probably a mixture of lead silicate and basic lead sulfate since analyses of individual areas of this phase tend to vary appreciably from area to area. In contrast, the analyses of individual areas of the lead-zinc-silicate phase show little variation and are essentially equivalent to PbZnSiO4. Only one analysis was made on the very dark phase within the lead-zinc-silicate and the composition approaches that of Zn2SiO4. The sulfur in the melt is not associated with the zinc.
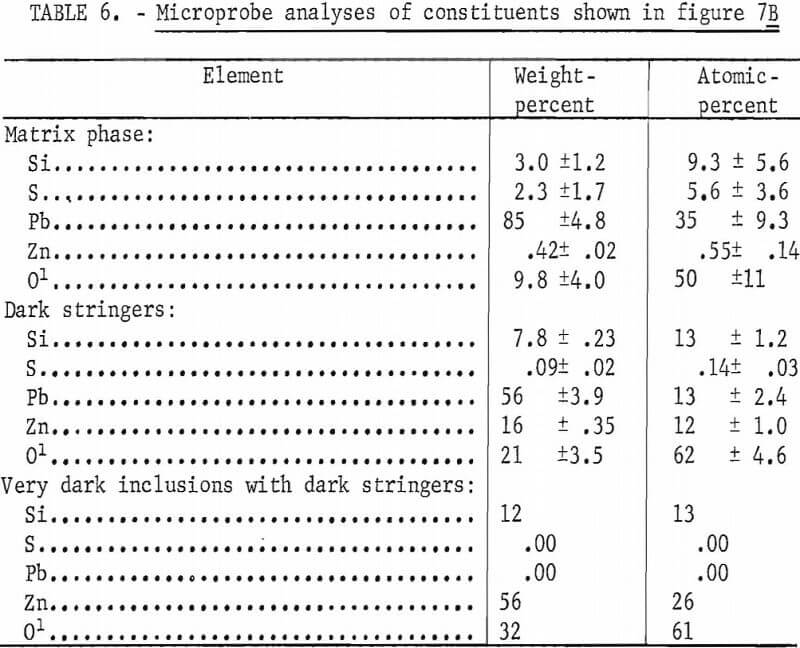
Lead-sulfide dendrites have only been observed in sections made from mixtures of PbS-ZnS and SiO2 and only then in mixtures containing 6 pct or more silica. Lead-sulfide dendrites were not observed in sections containing calcium additions. Examination of the micrographs show that the lead-zinc -silicate phase is the first phase to form during cooling of the melt and that the lead sulfide dendrites form during the freezing of the matrix which still contains appreciable sulfur after removing a portion as lead sulfide.
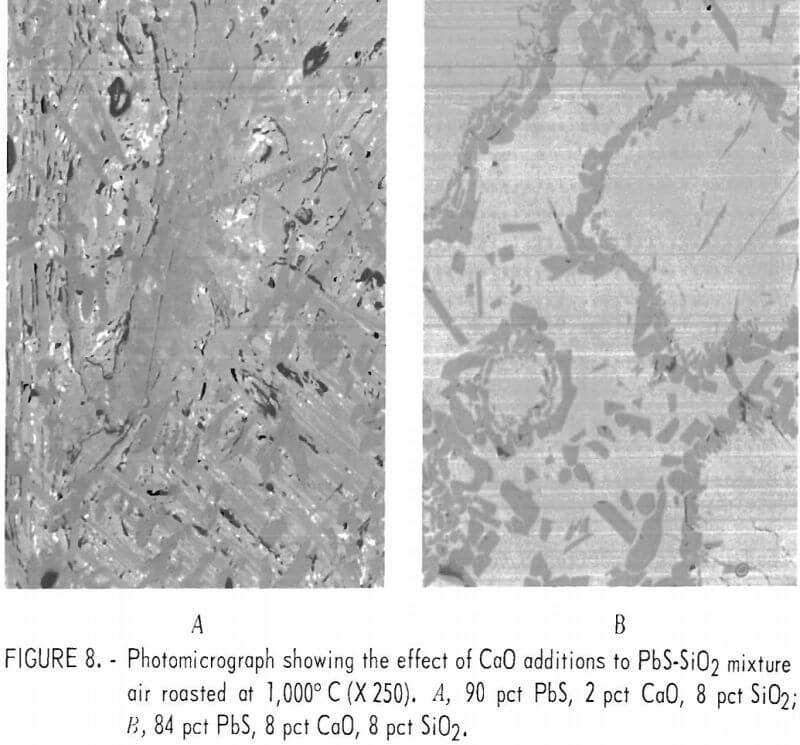
PbS-CaO-SiO2
Figure 8A shows a melt produced from a mixture consisting of 90 pct lead sulfide, 2 pct lime, and 8 pct silica. Figure 8B is a melt from 84 pct lead sulfide, 8 pct lime, and 8 pct silica. Table 7 gives the results of a microprobe analyses of the matrix material (dark gray) shown in figure 8A and the results indicate that the matrix is lead silicate (2PbO·SiO2). However, the calcium-and sulfur-bearing phases are too finely intermixed to make meaningful microprobe analyses since we could not analyze regions smaller than approximately 10 microns.
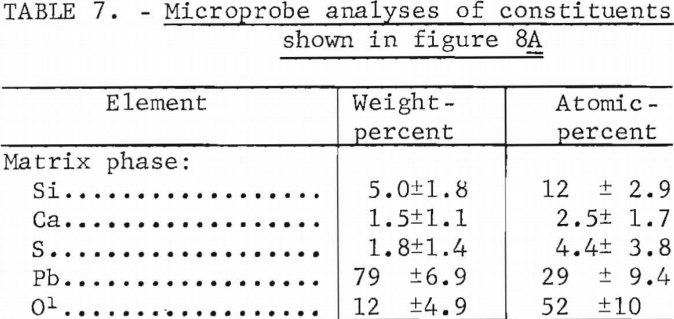
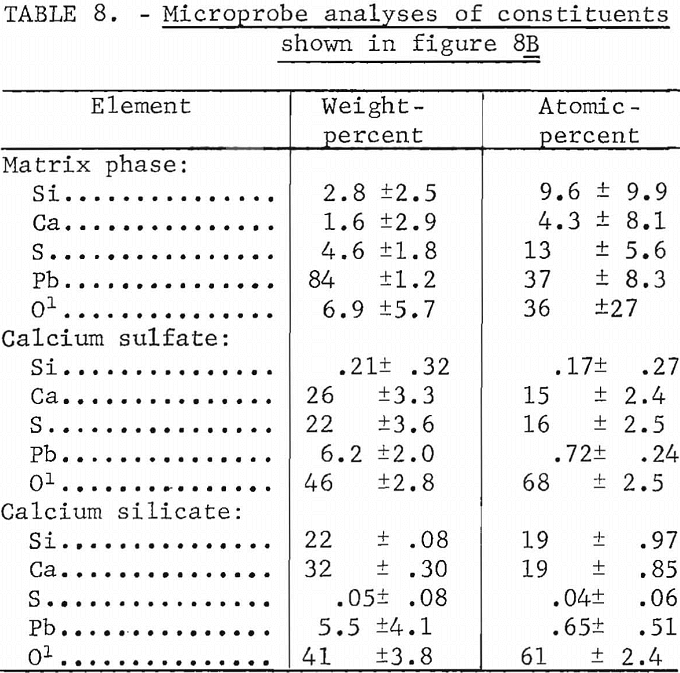
Microprobe analyses of the mixtures containing 8 pct lime and shown as figure 8B are listed in table 8. Several areas such as shown in the upper left-hand corner of figure 8B consisted of calcium silicate. The composition of the matrix phase is variable as shown by the microprobe analyses. It is probably a mixture of silicate and sulfate although under the microscope it appears as a single constituent. The microprobe analyses of the calcium sulfate and calcium silicate phases also are shown.
PbS-CaO-ZnS
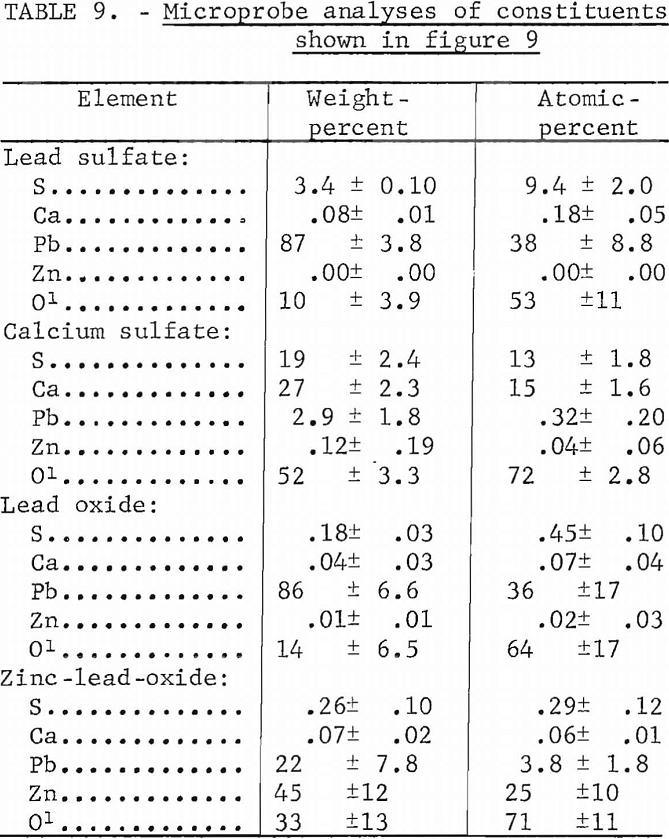
Two mixtures containing lime, lead sulfide, and zinc sulfide were prepared and heated at 1,000° C for 1 hour in an oxidizing atmosphere. The first mixture consisted of 4 pct CaO, 94 pct PbS, and 2 pct ZnS and the second contained 8 pct CaO, 90 pct PbS, and 2 pct ZnS. The microscopic appearance of these melts were very similar as shown in figures 9A and 9B.
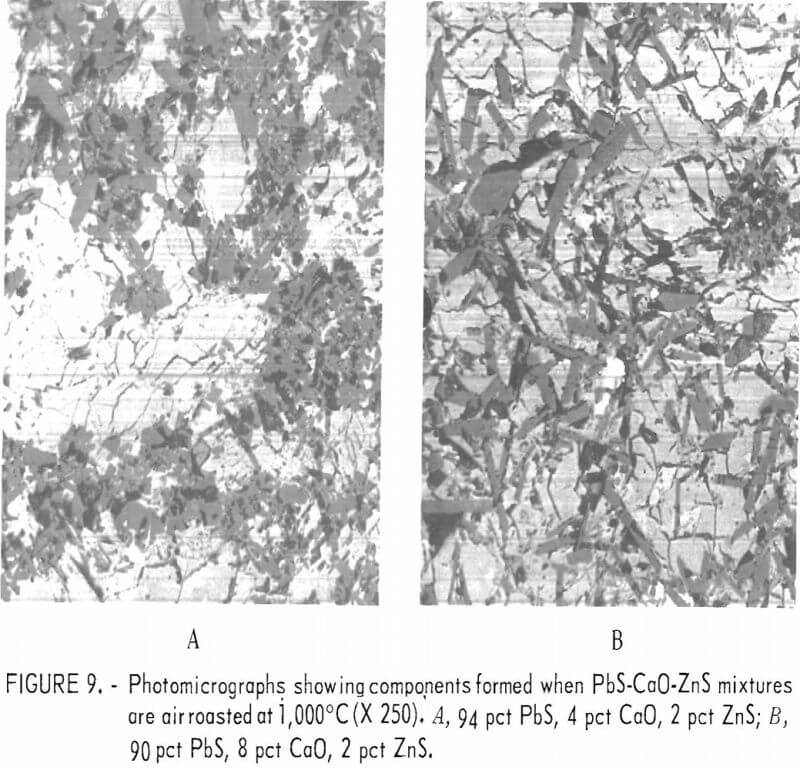
Micrographs of sections of these silica-free materials show that these materials do not resemble any of the sections containing silica. Quantitative microprobe analyses on the four constituents within the 8 pct CaO melt (fig. 9B) are given in table 9. The light colored matrix shown in figure 9B is identified as a basic lead sulfate. The dark inclusions are calcium sulfate while the very light intergranular material within the light matrix consists of a lead oxide and a zinc lead oxide. The two oxides are similar in appearance in the micrograph.
PbS-ZnS-SiO2-CaO
Addition of zinc to the lead sulfide and silica mixtures resulted in formation of zinc-lead silicate crystals as shown earlier. When lime was added to the PbS-SiO2 mixtures containing 2 pct zinc sulfide, calcium sulfate crystals and inclusions were formed within the silicate matrix as shown in figure 10. The mixture contained 8 pct silica, 6.5 pct CaO, 1.9 pct zinc sulfide, and 83.6 pct lead sulfide. The zinc appeared to be more or less evenly distributed throughout the lead silicate matrix shown in figure 10A. This matrix also contains a small amount of calcium and sulfur given in the first analyses in table 10. The second analyses are that of CaSO4 crystals.
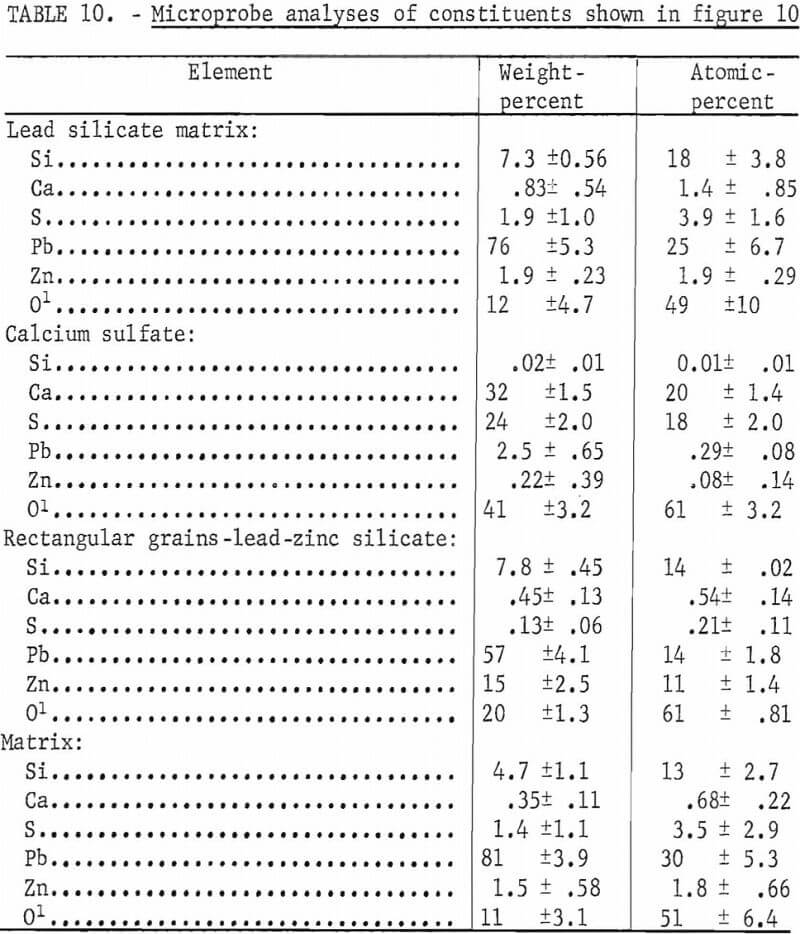
Figure 10B shows a micrograph of a section from a melt containing 79.2 pct PbS, 6.5 pct CaO, 8.0 pct SiO2, and 6.3 pct ZnS. The mixture contains 5 pct higher zinc than the mixture shown in figure 10A. Calcium sulfate crystals are readily apparent in the micrograph but in addition, the elongated rectangular crystals of Pb-Zn silicate were present also. The third microprobe analyses shown in table 10 are of these rectangular crystals. The fourth analyses are of the matrix phase which consists primarily of lead and silica with small amounts of zinc and sulfur. The micrograph shows that the matrix consists of two phases neither of which displays sufficient area to focus the electron beam for an analysis.
Under the conditions of our experiments we did not form calcium silicate. Apparently calcium silicate cannot form until sufficient silica is present to satisfy the requirements of the lead and zinc oxides to form silicates. We did not determine at what level this requirement would be met. The effect of adding calcium silicate to the mixture rather than calcium oxide was not determined although this could have an important bearing on the problem since half of the calcium in sinter is recycled as silicate present in the returned slag and sinter.
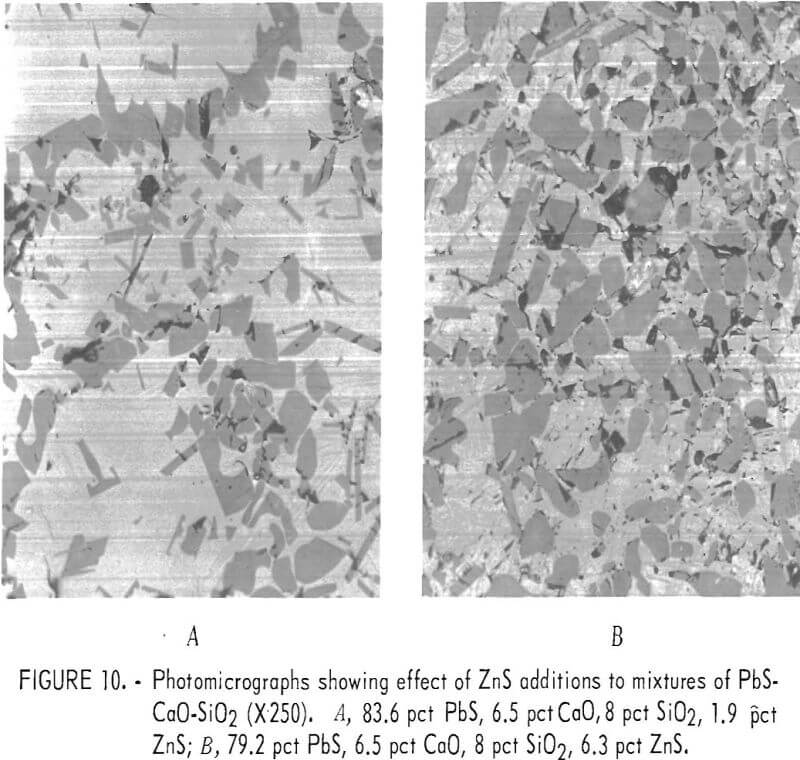
Figure 11 shows X-ray scans on an area from the melt shown in Figure 10A which demonstrates the distribution of the various elements within the section. Calcium and sulfur occur together and lead, silicon, and zinc occur together. The sulfur scan, although not shown in the figure, corresponds with the calcium scan. The areas showing calcium also show sulfur and those not showing calcium give little if any indication of sulfur.
PbS-SiO2-ZnS-Fe2O3
Iron oxide additions to mixes of PbS, SiO2, and ZnS were studied. Fine-ground hematite and PbS were mixed with 6 pct SiO2 and 2 pct ZnS. Figure 12 shows micrographs of two mixtures after being oxidized at 1,000° C. Figure 12A is of a mixture containing 1 pct Fe2O3 and 91 pct PbS. Figure 12B is from a mixture with 3 pct Fe2O3 and 89 pct PbS. The mix containing 1 pct Fe2O3 appears to be a fine-grained mixture of lead silicate and basic lead sulfate with finely divided ferrite. The microprobe analyses of the ferrite and average analyses of the matrix silicate phase are given in table 11. Also given are the analyses of the larger ferrite grains and the lead silicate glass matrix formed with 3 pct Fe2O3 additions as shown in figure 12B. The microprobe analyses of both the fine and the coarse ferrites are shown to be essentially the same. The silicate matrix phase in both samples was mostly lead silicate but iron, zinc, and sulfur were present.
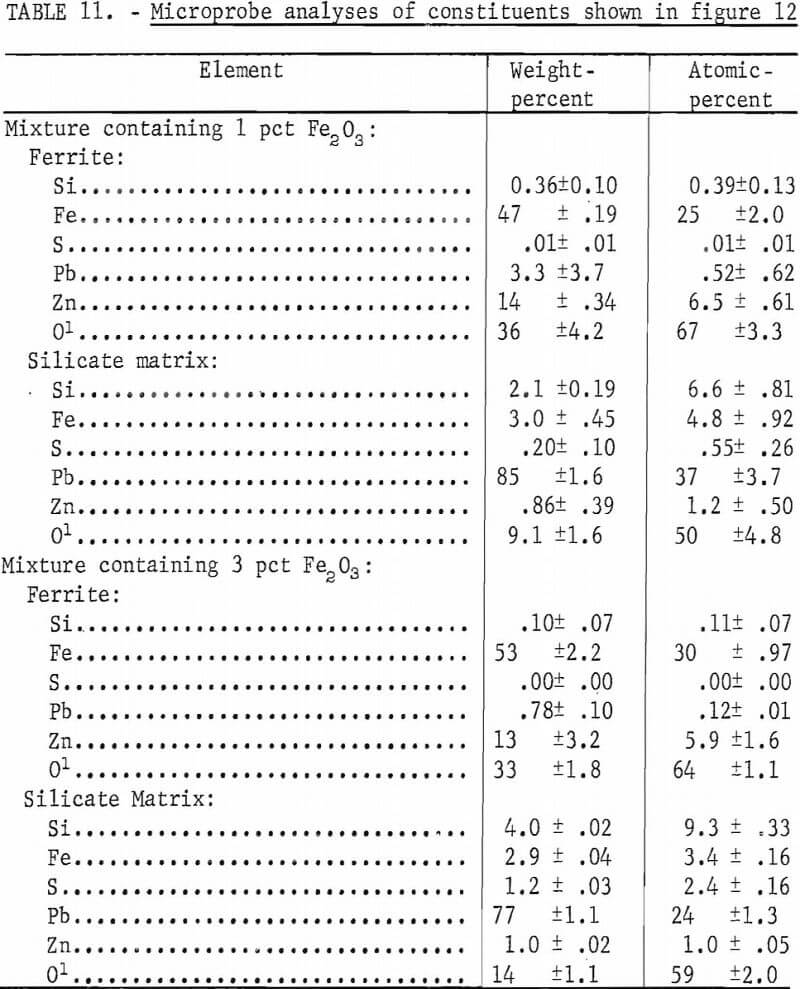
On increasing the zinc content of the mixture while leaving the iron and silica content the same, the formation of distinct lead-zinc silicate crystals was evident with ferrite crystals also occurring. Figure 13A is a micrograph showing a section from near the top of a melt prepared from a mixture of 87 pct PbS, 6 pct SiO2, 4 pct ZnS, and 3 pct Fe2O3. Figure 13B is of a section from the bottom of the same melt. The top section shows stringers of lead zinc silicate in a lead silicate matrix and the bottom section shows the ferrite crystals which formed or settled to the bottom of the melt during cooling.
Discussion and Conclusions
Lead sintering is a process which involves a complex set of interactions of all constituents in the mixture being sintered. It is not a simple Pb-S-O reaction with other materials being added for dilution purposes. Lead sinters are composed essentially of lead silicate 2PbO·SiO2, a calcium and zinc-bearing lead silicate (Pb, Ca, Zn)SiO2, zinc-bearing calcium silicate (melilite), and a ferrite or spinel containing both zinc and aluminum [(Fe, Zn)O·(Fe, Al)2O3]. Lead and lead sulfide are present and lead sulfates and oxides occur only sparingly in good sinters. Lead sulfates and oxides occur primarily as a result of nonhomogeneous mixing which prevent lead oxide from contacting silica.
Lead concentrates are difficult to sinter without additives containing silica. High sinter bed temperatures obtained when attempting to sinter undiluted concentrates result in the vaporization of much lead sulfide and oxide. In addition, the sinters burn unevenly producing bypassed areas of unsintered material and in some areas much lead metal is formed by this roast reduction reaction. With the vaporization of much lead and the producing of lead metal, the remaining material becomes more concentrated with nonvolatile impurities such as silica, calcium, zinc, and iron which react with the lead oxide to form products identified in commercial sinter.
Mixtures of PbS with 1 or 2 pct SiO2 heated in air to 1,000° C form lead oxide, lead sulfate and lead silicate, and some lead metal. Increasing the silica content to 4 pct or 8 pct of the mixture produces melts free of lead oxide or basic lead sulfate and results in a two-phase basic lead silicate system with both phases containing up to 1.5 wt-pct of sulfur.
Addition of 2 weight-percent zinc sulfide to lead sulfide containing 1 or 2 pct SiO2 results in zinc oxide and zinc silicate being formed by heating in air to 1,000° C. Mixtures containing 6 and 8 pct silica and 1 to 2 pct zinc sulfide form glassy lead silicate melts containing needlelike inclusions of lead-zinc silicate and dendrites of lead sulfide. Lead sulfide dendrites were only observed in lead silicate melts containing zinc. Increased zinc additions result in more lead-zinc silicate formation and there is indication that zinc silicate will be formed under some conditions.
Lime added to lead sulfide-silica mixtures and heated in air to 1,000° C results in melts containing calcium sulfate and lead silicate. As the lime content of the mixture is increased to about 8 pct calcium with silica remaining at 8 pct and the lead sulfide content is reduced to 84 pct, the melts show calcium silicate also being formed.
Lime, lead sulfide, and zinc sulfide mixtures heated in air to 1,000° C do not resemble any of the melts containing silica. In these mixtures virtually all of the lead is present as basic lead sulfate and lead oxide and zinc oxide containing lead.
Lime, lead sulfide, zinc sulfide, and silica mixtures treated similarly to previous samples show that calcium has a strong affinity for sulfur to form calcium sulfate, and the lead and zinc oxidize and combine with silica. Calcium silicates were not formed when CaO content was 6.5 weight-percent and the SiO2 content was 8 weight-percent. Evidently, calcium silicate does not form until sufficient silica is present to satisfy the requirements of the lead and zinc.
Iron forms ferrite in melts containing zinc. If insufficient iron is present in the mixture to combine with the zinc to form ferrites, the excess zinc will combine with lead and silica to form a distinct lead-zinc silicate component. Small amounts of iron and zinc are present also in the lead silicate matrix.

