Table of Contents
The purpose of this investigation was to determine the flotation characteristics of important columbium / tantalum ore minerals as a guide for the practical concentration of low-grade columbium ores.
Placer deposits amenable to gravity concentration have been the major source of columbium; however, these deposits are limited. Therefore, the future of columbium is dependent upon exploiting the large reserves of low-grade ores. Effective beneficiation of many of these complex ores requires mineral separations in the flotation size range. Current commercial flotation of columbium minerals is limited almost entirely to the processing of pyrochlore at Oka, Quebec, Canada. This study was undertaken to add to the growing but still limited information on the flotation characteristics of columbium minerals, and thus aid in the development of resources to meet future expanded demands for the metal. These future demands will almost certainly result from expanded uses in electronic and magnetic equipment and in refractory applications. However, the demand of greatest volume can be expected from increased use in steels and in alloys, both ferrous and nonferrous.
The experimental techniques used in this investigation were contact angle, microflotation, and bench-scale flotation. The first two methods were used to develop basic information on collector action which was utilized in bench-scale studies. Contact-angle results served as a guide in the microflotation study, and results of single-mineral microflotation studies were applied in bench-scale tests of complex ores.
Other investigations of columbium-mineral flotation have dealt principally with pyrochlore. The chief methods of investigation have been bench-scale flotation; other techniques used to a lesser extent include micro-flotation, adsorption, radioactive tracer, and bubble pickup methods. Except for the need to modify the contact-angle technique, the results of this study were in general agreement with those reported previously.
Contact-Angle Studies
The contact-angle method was used for the initial phase of this investigation because the number of factors influencing the collector-mineral relationship are minimized, and a comparatively rapid scan of the collector-mineral relationship can be made.
Equipment, Materials, and Test Procedure
A free-bubble contact-angle apparatus, similar in design to McGlashan’s, was used in this investigation. Reaction bath temperature and pH were monitored continuously and held within close limits; temperature ±1° C, pH ±0.05 unit.
Specimens were cut and polished using conventional equipment and materials but were handled to minimize contamination and alteration during preparation. Water was used as a lubricant. The criterion for the final polished surface was apparent freedom from scratches when viewed at 100 times magnification. Reliable cleaning of the specimen test face was accomplished by buffing on a cloth flooded with 0.5-micron alumina-water slurry.
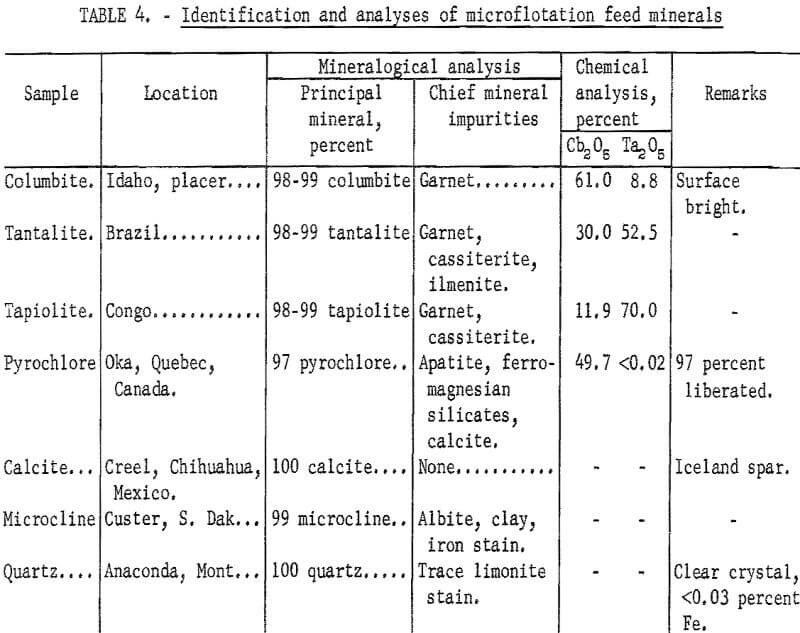
Emphasis was directed toward study of the columbium-bearing minerals, columbite, pyrochlore, tantalite, and tapiolite. The gangue minerals, calcite, microcline, and quartz, were studied to a lesser extent. All specimens were identified petrographically and, where necessary, were further examined chemically and by X-ray diffraction. With the columbium-tantalum minerals it was necessary to make mineral identification on the basis of chemical analysis because normal Fe and Mn content may result in an indefinite X-ray pattern. In instances where X-ray identification differed from that based on chemical analysis, as with columbite-tantalite minerals, the chemical identification was used because it provided more positive identity. Descriptions and identification of the specimens were used in this study and are reported in table 1.
Before contact-angle measurements were made, each specimen was tested in distilled water for evidence of bubble adherence. In this test, a captive bubble was pressed against the specimen face. If any sign of bubble adherence was observed, the specimen was recleaned and retested.
Contact-angle test procedure and calculations of contact angle followed those used in the free-bubble method except the bubble was pressed against the area to be tested before the bubble was released or withdrawn.
With the minerals and reagents tested, this pressure modification resulted in reduction of contact-angle variability and improved reliability of the data. However, contact angles observed tended to be higher than with the “free-bubble” method as described by McGlashan. Contact angle observed on lucite agreed with values found by Gayle by the captive-bubble method.
Specimens were conditioned prior to testing by suspending the mounted specimen in a beaker of test solution which was stirred by a rotating plastic-covered magnet.
Three qualitative terms, “no contact,” “cling,” and “threshold” are used in this report to supplement the numerical data and to aid in defining observed conditions.
The first term, “no contact,” abbreviated NC, denotes bubble-mineral attraction is nil; the second term, “cling,” is used to describe the condition of bubble-mineral attraction great enough to distort the bubble slightly but without indication of possible bubble attachment; “threshold,” abbreviated Thres, denotes a condition of bubble attraction significantly greater than “cling,” but one not resulting in ready bubble attachment.
Contact-Angle Results
Results of the contact-angle study are reported in three parts: anionic reagents, cationic reagents, and 8-quinolinol. Anionic reagents investigated were alkyl sulfate, alkyl sulfonate, alkyl phosphate, and fatty-acid reagents; cationic reagents included primary, tertiary, and quaternary amines. Reagent concentrations reported are actual concentration of active compounds. Significant contact was found on ore minerals with alkyl phosphate and most of the amine reagents under a variety of conditions. With reagents of the fatty-acid or petroleum-sulfonate types, contact angles were observed only when the two types were used together as a combined reagent. Contact angles with a tertiary amine and 8-quinolinol were either insignificant or marginal.
Sodium oleate was investigated as a collector for columbite, tantalite, and to a lesser extent, as a collector for pyrochlore, calcite, microcline, and quartz. At room temperature no contact was observed on clean surfaces of columbite or tantalite specimens in solutions containing sodium oleate alone. Columbite was tested through the range pH 2.5 to 10 in 100 mg per liter sodium oleate. No contact was obtained on tantalite, microcline, or quartz. Also, no contact was obtained with columbite or tantalite at pH 6, 8, and 10 in 300 mg per liter sodium oleate solution. Nor was contact obtained with columbite, pyrochlore, or quartz when tested at pH 8 in 20 mg per liter solution. Similar action of sodium oleate has been reported by others.
However, when a technical-grade oil-soluble alkyl sulfonate was used as a reagent partner with sodium oleate on a 1:1 weight basis, significant contact angles were obtained at pH levels below 7 on columbite, tantalite, and pyrochlore. These data are shown in table 2. Similar results were obtained with columbite when a water-soluble alkyl sulfonate was substituted for the oil-soluble alkyl sulfonate.
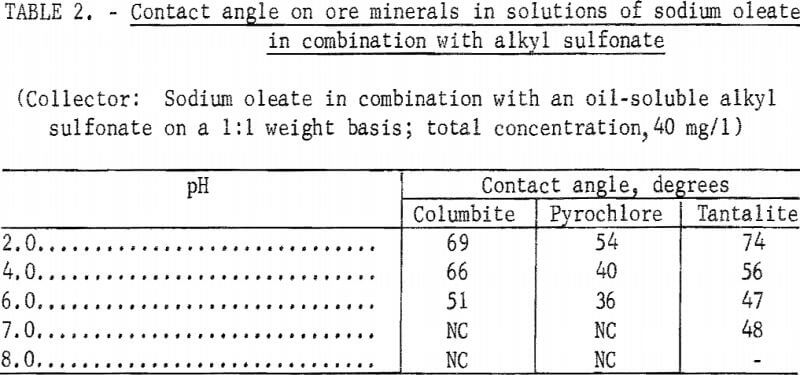
Note.-NC means “No contact.”
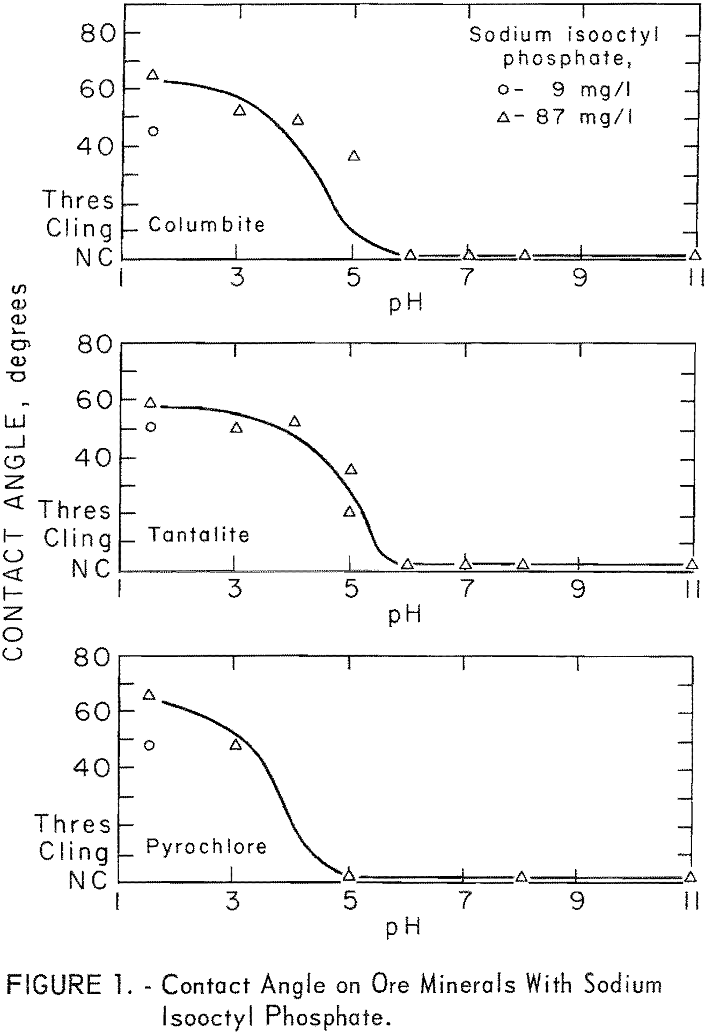
Sodium isooctyl phosphate was the most effective reagent of four sodium organic phosphates tested at pH 3. The other organic groups in these reagents were ethy 1, 2-ethyl hexyl, and phenol. Figure 1 shows the. effect of pH levels on ore-mineral contact angle for 87 mg per liter sodium isooctyl phosphate. No contact angle was observed on quartz or microcline in the pH range 1.5 to 11 at collector concentrations of 87 and 175 mg per liter. A contact angle of 51 degrees was obtained on calcite at pH 7 with 175 mg per liter sodium isooctyl phosphate.
Contact angles on columbite, pyrochlore, and tantalite were investigated in solutions containing sodium 2-ethyl hexyl sulfate and sodium tetradecyl sulfate at 100 mg per liter concentration. Three pH levels- 2, 4, and 8- were investigated. The only significant contact angle obtained was 49 degrees on columbite at pH 2.0 with sodium tetradecyl sulfate.
Amine reagents studied included primary, tertiary, and quaternary types. The primary and quaternary amines demonstrated collecting action in alkaline solutions at low concentrations of reagent.
Primary amines studied were n-dodecylamine acetate with a n-dodecylamine acetate content of more than 99 percent and two long-chain primary amines, One, known as n-octadecenylamine, had an amine content of 30 percent hexadecyl, 25 percent octadecyl, and 45 percent octadecenyl; the other, known as n-octadecylamine, was 6 percent hexadecyl, 93 percent octadecyl, and 1 percent octadecenyl.
The n-dodecyclamine was studied through a collector range of 3.75 to 250 mg per liter and through a pH range of pH 2 to 12. Some of the data given in figure 2 shows no significant difference for contact angle on columbite with 3.75 mg per liter n-dodecylamine acetate used alone or used with 1.35 mg per liter sodium dodecylbenzene sulfonate. Also in figure 2 are reported results on pyrochlore and tantalite with the same reagents.
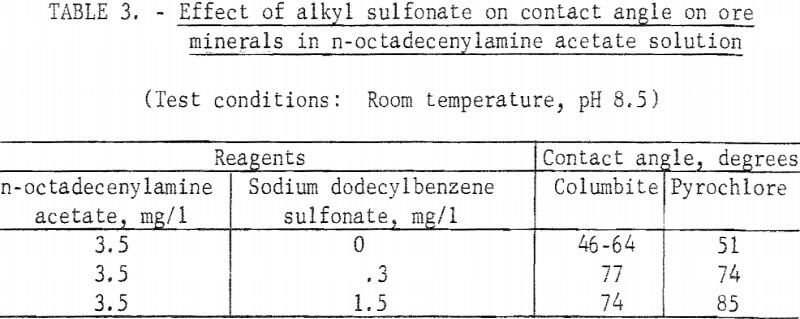
The two long-chain primary amines were investigated briefly for collecting action at pH 8.5 each, alone and with an anionic surfactant, Sodium dodecylbenzene sulfonate was used in conjunction with the n-octadecenylamine and an anionic fluorosurfactant was used with the n-octadecylamine. Both amines produced significant contact angles on eolumbite, pyrochlore, and tantalite. A significant increase in contact angle resulted when these long-chain amines were used with anionic surfactants. Results with n-octadecenylamine acetate with an alkyl sulfonate are reported in table 3.
Tertiary amine, 1-hydroxyl-2-heptadecenyl glyoxalidine, was investigated at a concentration of 100 mg per liter. Columbite was surveyed at pH levels 2, 4, and 8 and tantalite at pH 6 only. The only contact angle observed was a marginal 36 degrees at pH 8 on columbite.
The majority of the investigation of quaternary collectors was with stearyl dimethylbenzyl ammonium chloride. At 2.5 mg per liter, significant contact angles were observed on columbite, pyrochlore, tantalite, microcline, and quartz. No measurable contact angle was observed on calcite in the range of pH 7.5 to 10. The contact angles observed on the ore minerals and on quartz are plotted in figure 3. Those for microcline were similar in value to
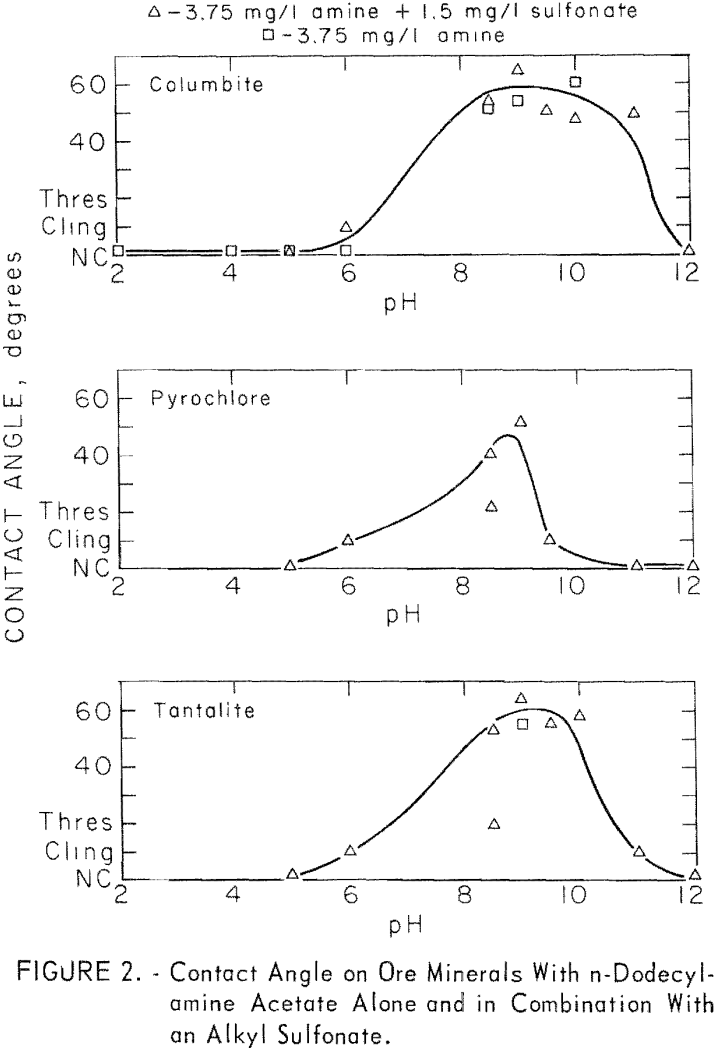
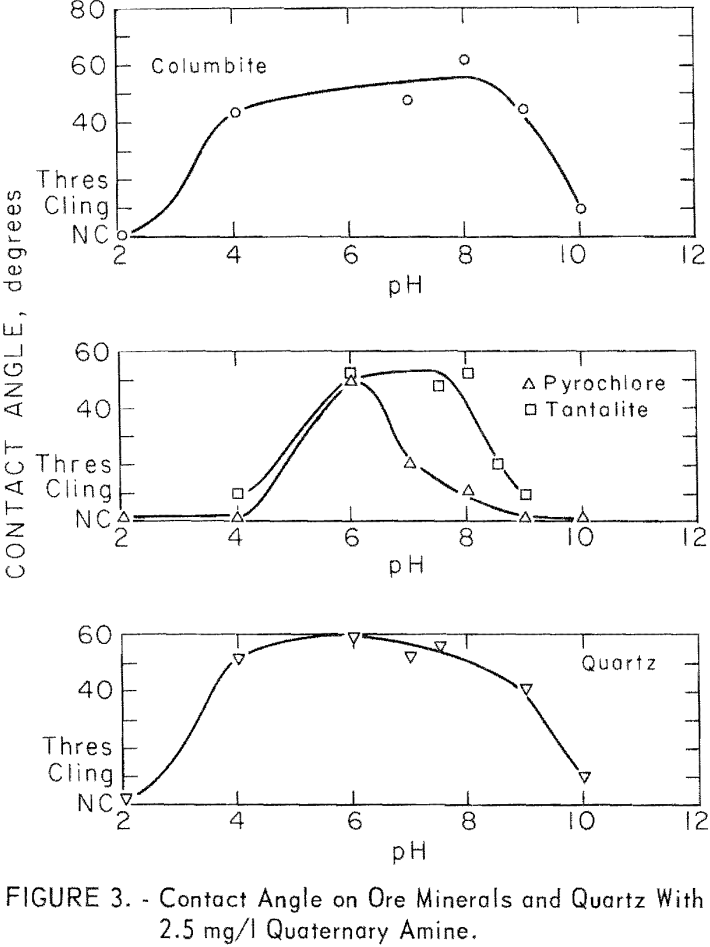
those of quartz. For microcline at pH 6, 8, and 8.5, contact angles were 58, 52, and 54 degrees. In general, the responsive minerals developed the highest contact angles between pH 6 and pH 9. Of the ore minerals, columbite developed the highest contact angles over the widest pH range and pyrochlore the lowest over the narrowest pH range.
Contact angles on columbite were investigated, at three concentration levels of quaternary amine chloride 2.5, 12.5, and 250 mg per liter.
No contact was obtained on columbite with 250 mg per liter collector concentration at pH levels of 2, 4, or 6. Double layering of the collector on the mineral surface may be the reason for this effect.
Exploratory surveys were made with 8-quinolinol in a water solution and in an emulsion. Patent literature reports columbium mineral flotation at pH 7.2 to 8.0. Significant contact angles were obtained only with an emulsion composed of 8-quinolinol and fuel oil emulsified with a nonionic surfactant. The respective reagent proportions were 100:67:4. When this emulsion was used at a concentration of 853 mg per liter, the contact angles observed on columbite were 51 degrees at pH 4, 54 degrees at pH 7.5, and 49 degrees at pH 10. On pyrochlore a contact angle was observed only at pH 4; it was 63 degrees. Columbite, pyrochlore, and microcline gave no evidence of contact angle at pH 5,7, pH 7.5, or pH 9 in a water solution containing 200 mg per liter of 8-quinolinol. At pH 9, calcite contact angle was 50 degrees.
Microflotation
Microflotation tests were used to extend information obtained in the contact-angle study. Both columbite ore minerals and common gangue minerals were tested.
Equipment, Materials, and Test Procedure
For the microflotation testing, a Hallimond tube similar to Iwasaki’s was used. It was modified to allow collection of successive concentrates during a test run. This was accomplished by attaching a hose to the end of the concentrate leg and extending the hose end above the surface of the cell solution. During flotation, a quick-release clamp closed off the hose to separate the two concentrates. Nitrogen passed through a CO2 absorbant was used for levitation. Small Pyrex glass dippers of appropriate volume were used to obtain the desired weight of mineral. Samples of wet 65/100 mesh quartz with an average weight of 1.35 grams showed a standard deviation of between 0.03 and 0.04 gram. Fuerstenau reports similar results.
From contact-angle observations 5 minutes was sufficient for columbium mineral conditioning. Preliminary microflotation tests showed most of the floatable mineral grains floated within 5 minutes. Therefore, a standard conditioning and flotation time of 5 minutes was used, with one exception.
Conditioning the mineral prior to flotation was accomplished in a cylindrical Pyrex flask of approximately 220-ml volume and placed in a “wrist-action” shaker. The conditioning tubes were about 8-½ inches long and 1-¾ inches ID; the bottom was hemi-spherical to impart swirling action.
Four ore minerals were selected to cover the range and type of minerals most likely to be encountered in practice: Columbite, pyrochlore, tapiolite, and tantalite. Gangue minerals selected were those most commonly associated with columbium-bearing ores; Calcite, microcline, and quartz. Each mineral was prepared and carefully separated from associated minerals by physical processes such as tabling, magnetic, and electrostatic separation. Petrographic, spectroscopic, and, where necessary, chemical, spectrographs, and X-ray methods were used for identification. Gangue mineral preparation was not difficult, for these minerals were obtainable with 1 percent or less impurities. Ore mineral preparation was less simple. However, purity of columbite, tantalite, and tapiolite prepared for use was 98 to 99 percent. Pyrochlore mineral purity was 97 percent; inclusions were the primary cause for the lower purity. Further information of each mineral lot is given in table 4. Included are the source, location, and chemical analysis.
After the minerals were sized and upgraded, the surfaces were cleaned by shaking the samples in distilled deionized water for ten 1-minute intervals each, followed by decantation. Each sample lot was then given a test for cleanliness by floating for 5 minutes in distilled water with no collector. Normally, two pH levels of 6.0 to 6.5 and 8.0 to 9.0 were checked. If the blank-float concentrate was more than 1 percent of the feed weight, the mineral was recleaned. After cleaning, the minerals were stored under distilled water in plastic bottles.
Reagents used in microflotation studies were chosen from those which showed potential as collectors in contact-angle studies. NaOH was the base used for pH adjustment and, unless specifically mentioned, the acid used was H2SO4.
After a standard conditioning time of 5 minutes, with collector, the conditioned mineral was transferred from the conditioning flask to the lower section of the Hallimond tube. Then, after checking pH and adjusting, if necessary, the remainder of the 160 ml of solution used in conditioning was introduced into the top of the assembled Hallimond tube and flotation started. During flotation, gas flow and stirring-magnet speed were held constant. Nitrogen flow for all tests was held at a meter value of 6.5 (70 ml per minute).
Standard flotation-recovery time for most tests was 5 minutes; however, because of the high flotation rate a time of 2 minutes was used for some amine tests. At the end of flotation, gas and stirrer were turned off and all mineral in the upper section of the cell was collected as final concentrate. An initial concentrate was collected after 30 seconds in flotation tests using sodium oleate or sodium oleate with oil-soluble sulfonate. With other collectors the initial flotation was 1 minute.
The initial concentrate weight exhibited greater variability than the final concentrate weight. However, flotation-rate information thus obtained was valuable in determining trends. All apparatus was cleaned with chromic-sulfuric acid cleaning solution and thoroughly rinsed in preparation for subsequent test work.
Microflotation Results
Tantalite was studied most extensively because of its availability, its abundance, and its intermediate position in the columbite-tantalite mineral series. Pyrochlore was studied the least extensively for two reasons: (1) A general ore-mineral behavior pattern had been already outlined by study of the other ore minerals and (2) the amount of pyrochlore material available was limited.
Anionic Reagents
Anionic collectors used in these microflotation studies were sodium oleate, sodium oleate with oil-soluble alkyl sulfonate, and sodium isooctyl phosphate. Also, hydrofluoric acid was studied briefly as an additional modifying agent for the oleate systems.
In the contact-angle study the combined reagent, sodium oleate-oil-soluble alkyl sulfonate (1:1 by weight), was more effective in obtaining a measurable contact angle on minerals than either sodium oleate alone or an oil-soluble alkyl sulfonate alone. Therefore, this combination reagent was selected for microflotation study. Tests were run to determine the effect of variation of pH and collector concentration levels as well as the effect of substituting HCl for H2SO4, as the pH-determining acid.
With this combined reagent, recovery of each of the ore minerals followed the same general pattern. This can be seen easily from the 5-minute recovery data in figure 4 for pyrochlore, tantalite, and tapiolite. In the pH range 2 to 10, recoveries showed two peaks, one near pH 2 and one near pH 8, with a
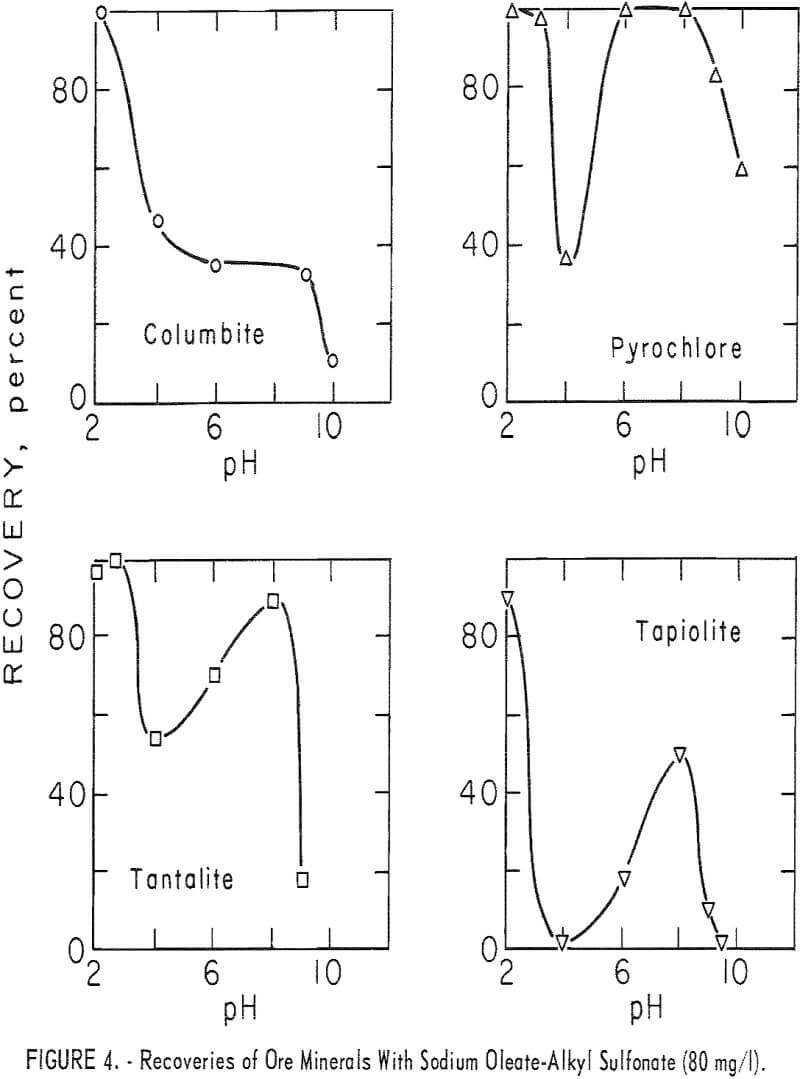
minimum point at 4. Above pH 8 recoveries dropped rapidly as pH increased. Columbite recovery was similar with a maximum at pH 2, dropping as pH increased. However, 1-minute flotation data showed an increase in flotation rate of columbite near pH 8, even though it was not discernable from the 5-minute recovery data. Data for collector concentration vs. recovery of three ore minerals at pH 8 are given in figure 5. No significant difference in recoveries resulted when HCl replaced H2SO4 as the pH-determining acid in the flotation of pyrochlore at pH levels of 2, 4, and 6 with 40 mg per liter of the combined sodium oleate-sulfonate collector. The combined reagent was also used to float the gangue minerals, calcite, quartz, and microcline. Calcite recoveries were complete through the pH range 6 to 10 at a collector concentration of 5 mg per liter. Respective recoveries of quartz and microcline for
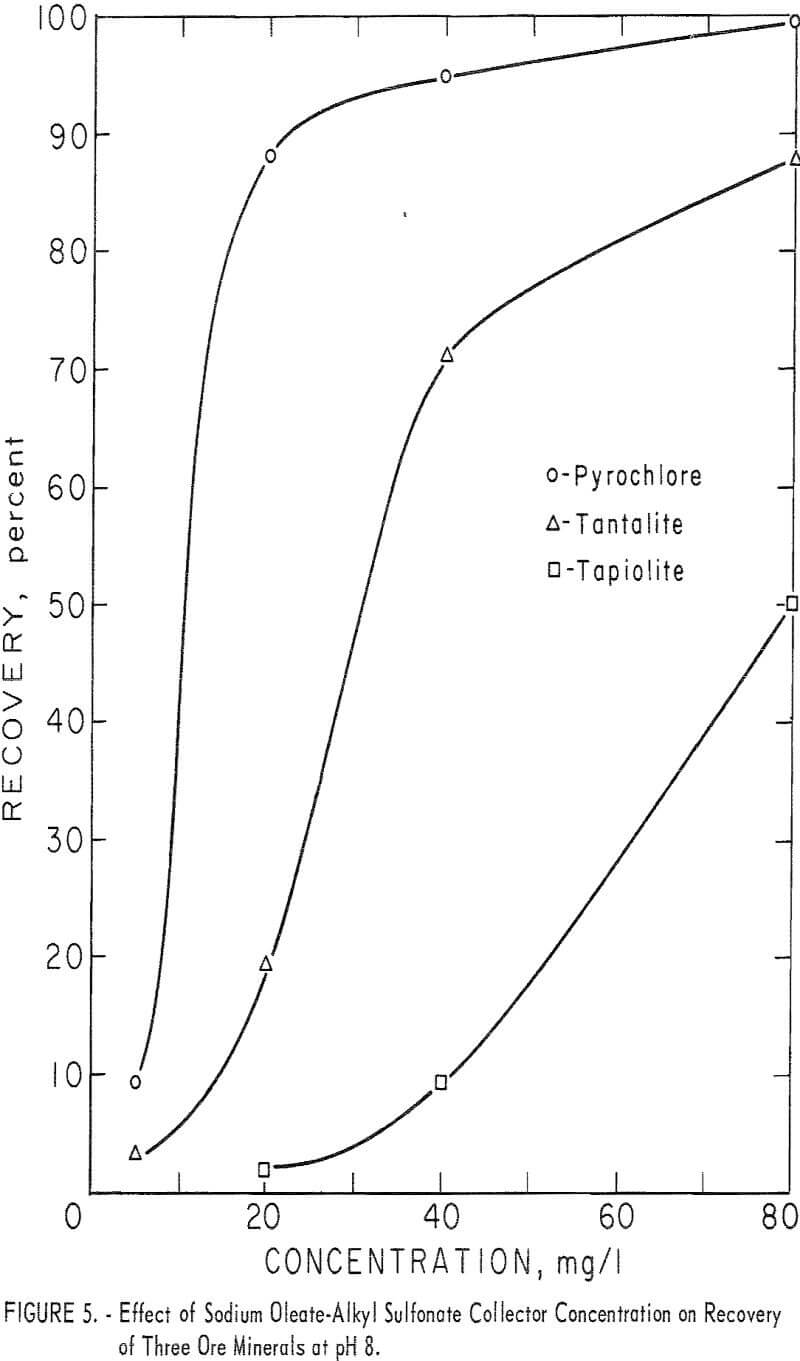
80 mg per liter total collector were 12 and 18 percent at pH 2 and 15 and 28 percent at pH 8. At the same concentration, as seen in figure 4, recoveries for the four ore minerals were 90 to 100 percent at pH 2 and 34 to 100 percent at pH 8.
Sodium oleate was investigated briefly because of its general use as a flotation collector and also because of the part it played as a partner in the combined reagent, sodium oleate-oil-soluble alkyl sulfonate. Flotation recoveries of columbite, tantalite, and pyrochlore were complete with 40 mg per liter sodium oleate at pH 8. Under the same conditions, tapiolite recovery was only 70 percent. Data for this test series are reported in table 5. Data showing the effect of hydrofluoric acid (HF) on the flotation of ore minerals are also reported in table 6. There is evidence that HF improves columbite flotation by cleansing the mineral surface.
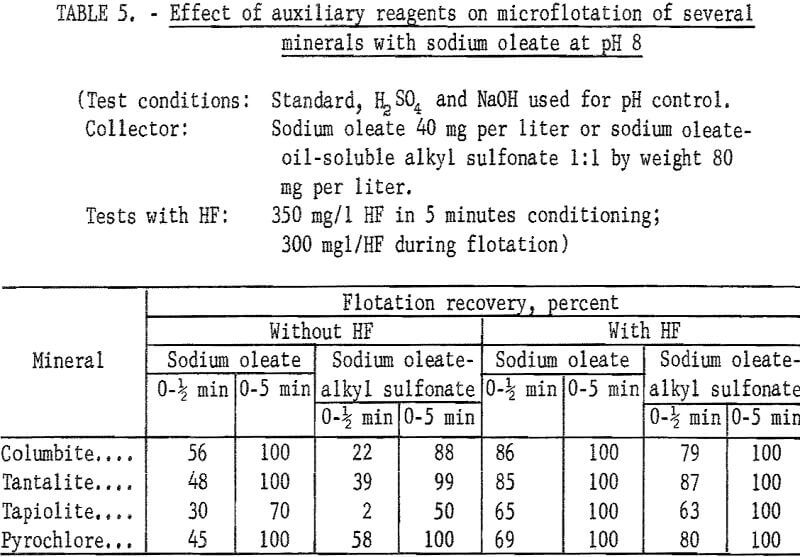
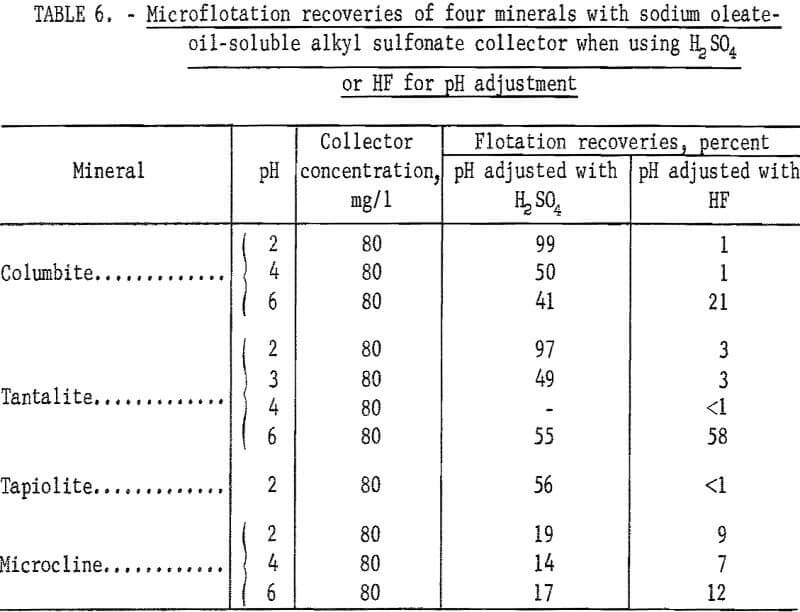
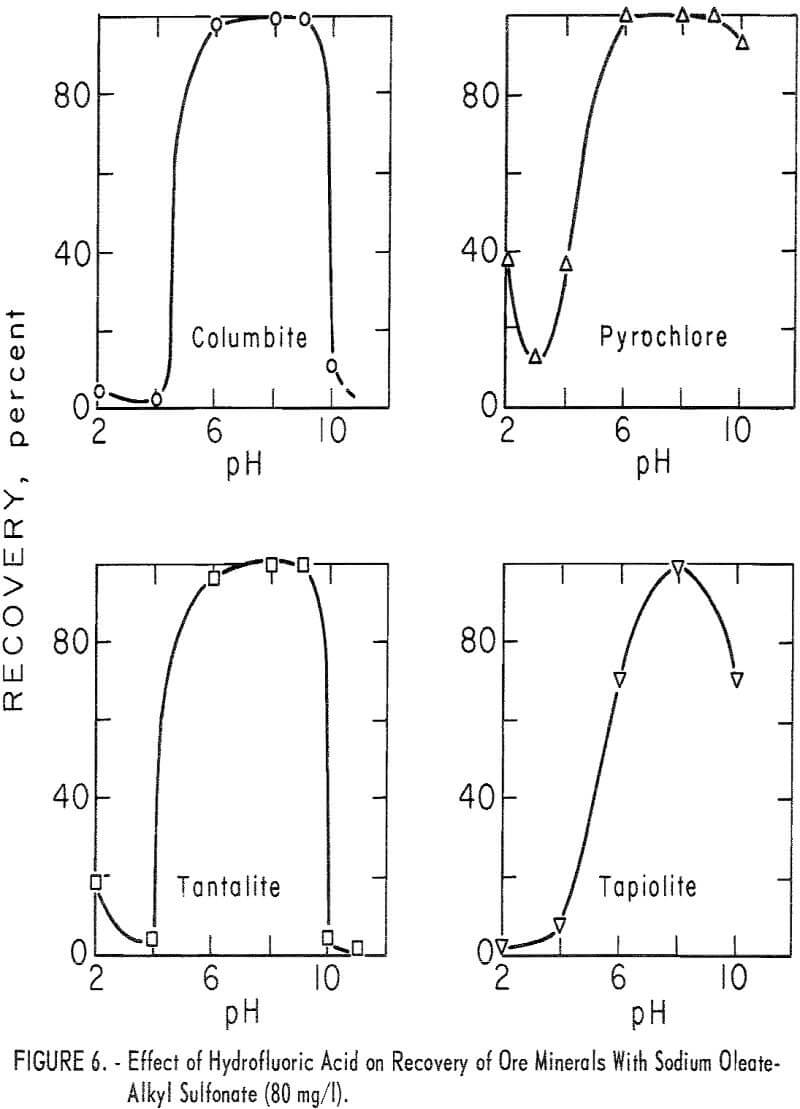
Use of HF in flotation or in conditioning and flotation resulted in a change in the recovery pattern of each mineral. If HF was used as the pH- determlning acid or if the products of HF preconditioning remained in the flotation liquor, both ore minerals and microcline were depressed at or below pH 4 (see table 6 for the effect of HF and H2SO4). However, with HF conditioning at pH 6 or above, ore-mineral flotation was enhanced. This is seen in the recovery-pH plot for the four ore minerals by comparing figure 6 with figure 4.
Sodium isooctyl phosphate was investigated as a collector for columbium-tantalum ore minerals because of promise shown in contact-angle tests. It was found to be an effective collector at low pH levels for each of the four ore minerals. Gangue-mineral collection at these pH levels ranged from low to
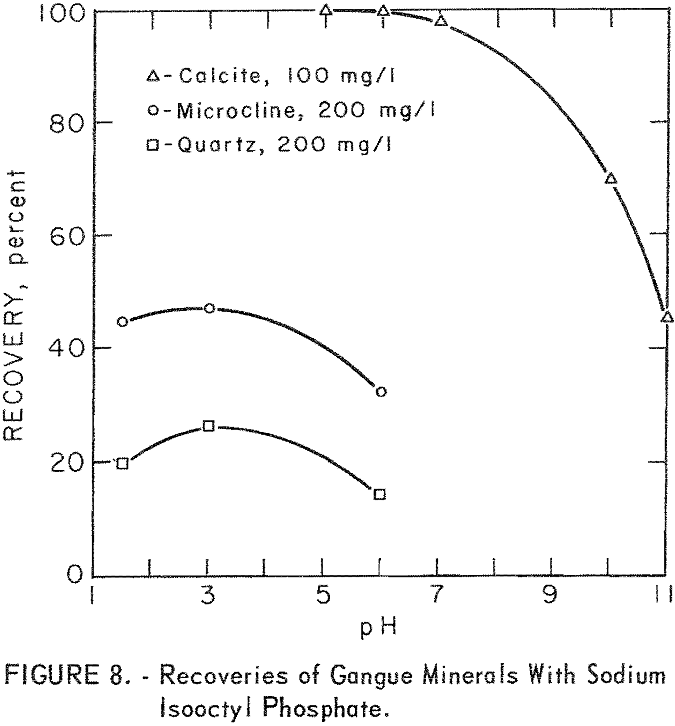
moderate; however, at higher pH levels (pH 5 to 7) calcite flotation was excellent. Pertinent data for ore minerals are shown in figure 7 and for gangue minerals in figure 8. These data show some selectivity between the ore minerals and quartz and microcline at low pH.
Recovery of ore minerals was complete at pH 3 with sodium isooctyl phosphate concentrations of 50 mg per liter. The recovery tended to decrease on either side of the optimum flotation level (near pH 3) of the ore minerals. This trend is illustrated by the 10 mg per liter recovery plots in figure 7. Pyrochlore showed these same trends, but recovery dropoff was less pronounced because of the more effective flotation of pyrochlore by sodium isooctyl phosphate. With 50 mg per liter sodium isooctyl phosphate, pyrochlore recovery 100 percent through the range pH 1.5 to 5. The collector-concentration
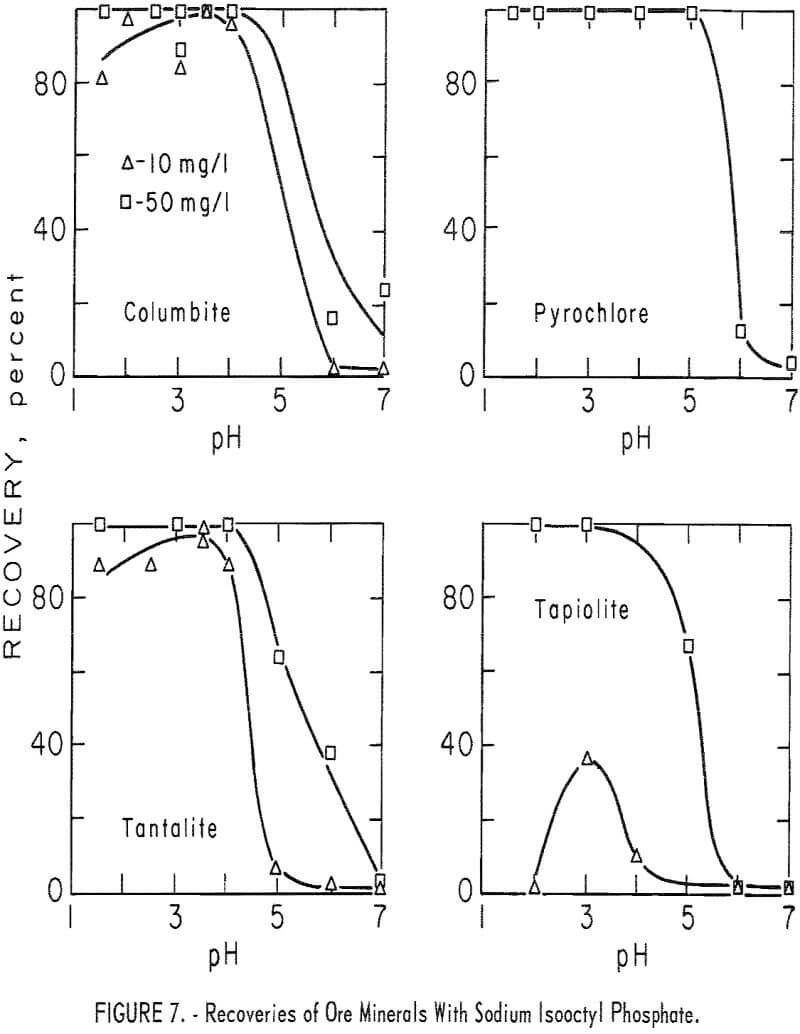
effect for columbite, tantalite, and tapiolite is shown by the 10 and 50 mg per liter curves in figure 7. Recovery of pyrochlore at pH 3 was 100 percent at both 50 and 100 mg per liter sodium isooctyl phosphate levels. At the same pH level and with 10 mg per liter the recovery dropped only 2 percent.
As may be seen in figure 8, recoveries of gangue minerals are considerably lower than recoveries of the ore minerals through the same pH range, even at the higher collector-concentration levels. For example, recoveries of microcline and quartz at pH 3 were 41 and 16 percent at sodium isooctyl phosphate concentrations of 100 mg per liter. The maximum recoveries of the siliceous-gangue minerals, microcline and quartz, at the highest collector concentration of 200 mg per liter, are, respectively, 47 and 27 percent. Calcite floated much more rapidly than the siliceous-gangue minerals at moderate pH levels. Recoveries of 100 percent were obtained with 100 mg per liter sodium isooctyl phosphate at pH 5 and 6, However, recovery dropped as the pH level increased above pH 6 or as the collector concentration decreased.
Cationic Reagents
Cationic reagents showed effective collector action in contact-angle study for ore minerals; therefore, representative amine types were investigated in these microflotation studies. Three cationic reagent types were studied. These included a short-chain primary amine, a long-chain primary amine, and a quaternary amine.
Limited studies of tantalite and quartz were made with the short-chain primary amine, n-dodecylamine. Tantalite was tested at five collector-concentration levels of 3.75, 7.5, 15, 30, 190 mg per liter and quartz at 3.75 mg per liter. Good flotation recoveries of both minerals were obtained. Tantalite recovery was 94 to 100 percent through the range pH 8 to 11 with 15 mg per liter collector. With 3.75 mg per liter, tantalite recoveries at pH 10 and 11 were, respectively, 91 and 94 percent. Quartz recovery was substantially complete through the range pH 7 to 11.5 with 3.75 mg per liter collector. These data are reported in figure 9.
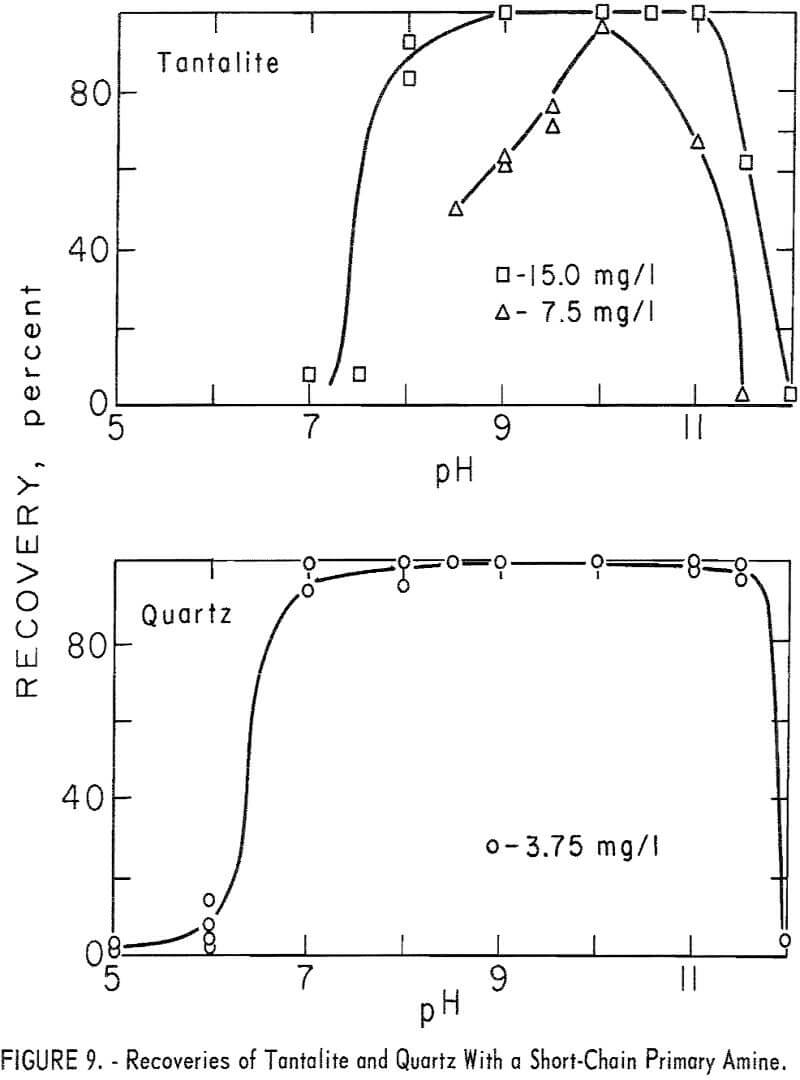
Flotation recoveries and pH stabilization were improved when sodium bicarbonate was used.
The long-chain amine, n-octadecenylamine acetate, was a strong collector for both ore and gangue minerals. Essentially complete recoveries of both ore and gangue minerals were obtained at collector concentrations of 5 mg per liter or less. Recovery data for columbite at 5 mg per liter and tantalite and tapiolite at 1 mg per liter are shown in figure 10. Tapiolite recovery
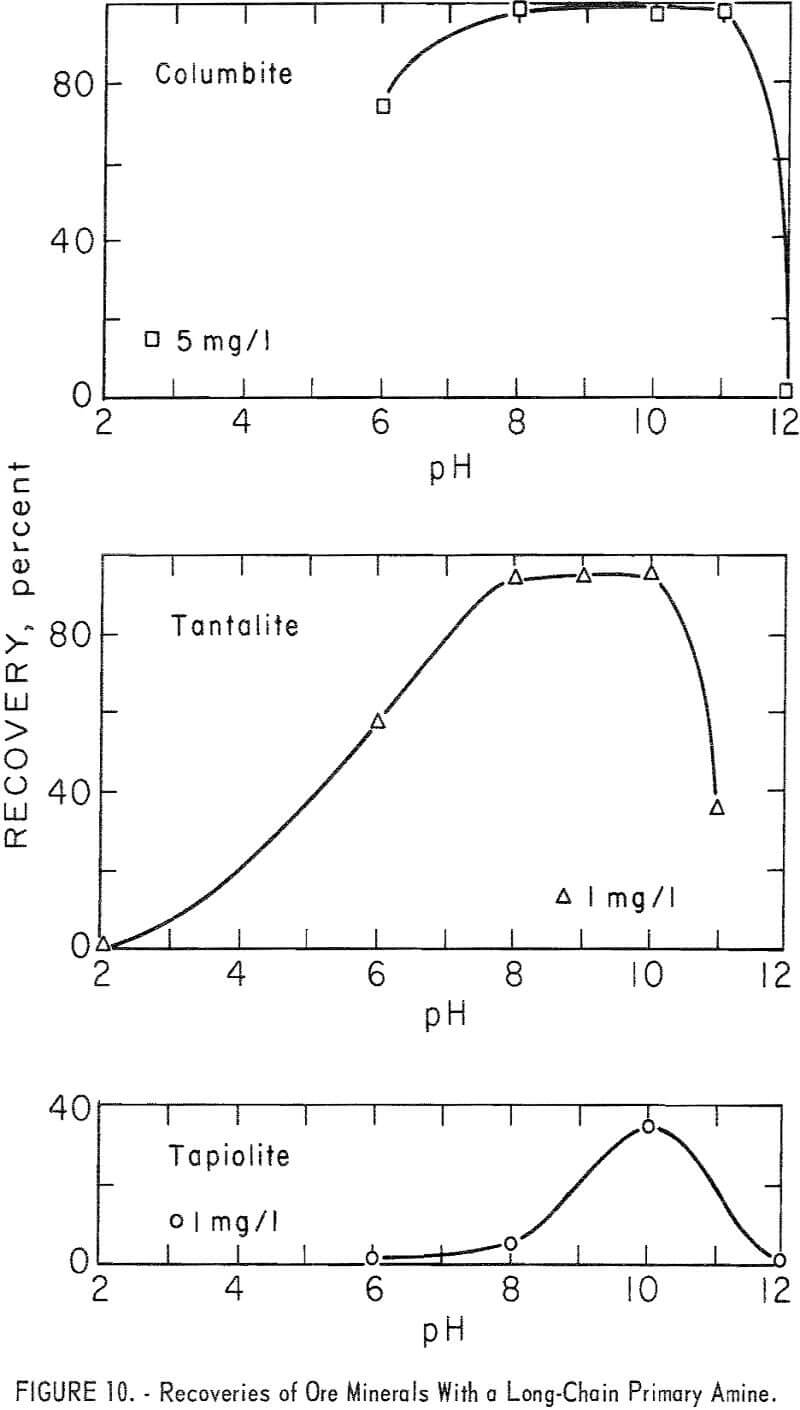
with 5 mg per liter of n-octadecenylamine acetate was 98 percent at pH 8 and 97 percent at pH 10. Pyrochlore recoveries at pH 8 and collector concentrations of 0.5, 1.0, and 5.0 mg per liter were 82, 97, and 100 percent respectively.
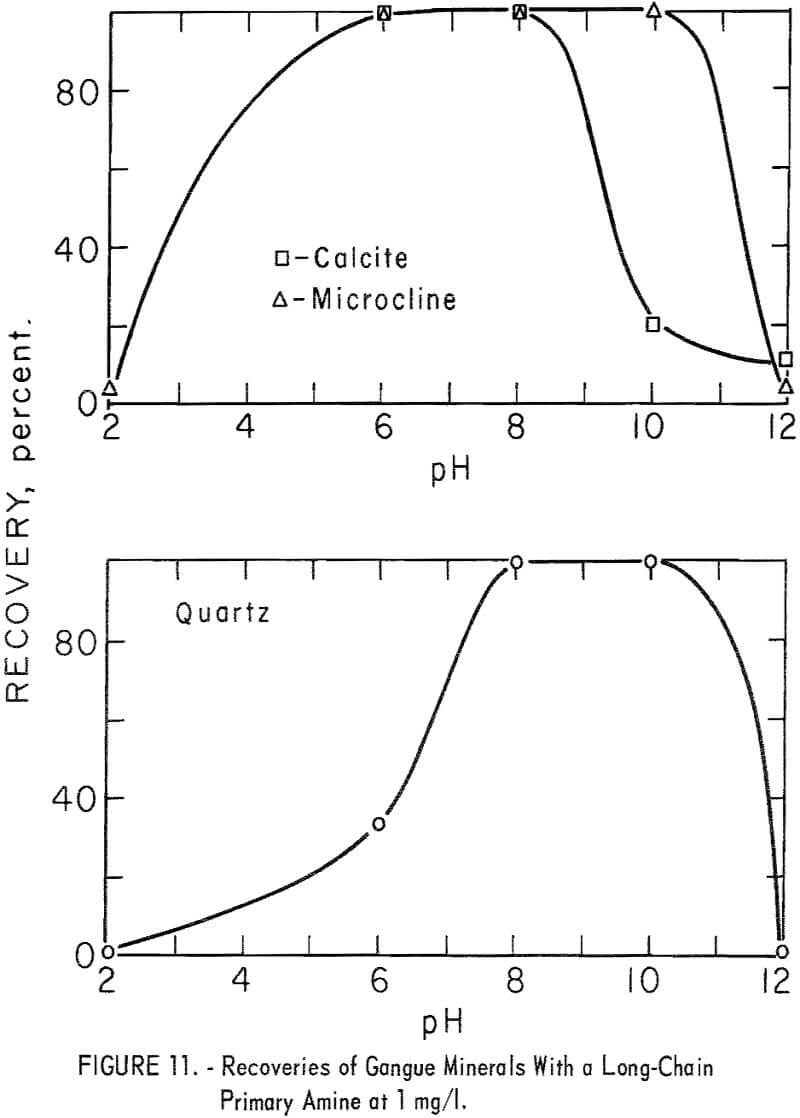
Data for gangue-mineral recoveries at 1 mg per liter are shown in figure 11. It will be noted that the best conditions for ore flotation also are good to excellent for gangue-mineral flotation.
Selective response of ore minerals and calcite was obtained in the contact-angle study using a quaternary amine, stearyl dimethylbenzyl ammonium
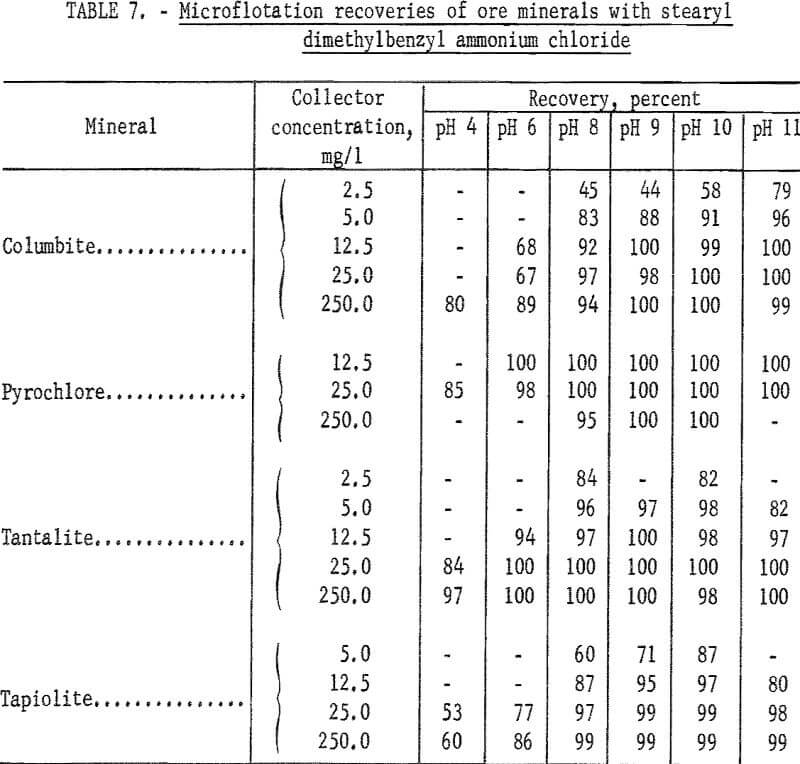
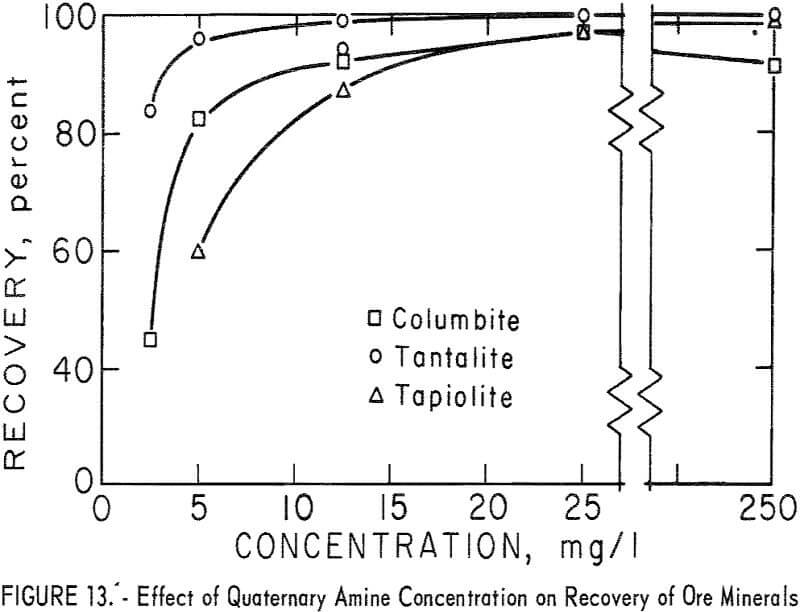
chloride. A microflotation study was made to obtain further information on the flotation characteristics of the ore minerals and of calcite, quartz and microcline. Optimum pH range for recovery of ore minerals was pH 9 to 10. Collector-concentration levels of 2.5, 5, 12.5, 25, and 250 mg per liter were studied. For the two highest collector-concentration levels, the principal pH range studied was pH 4 to 11. For the lower collector-concentration levels the principal pH ranges were between pH 6 and 11. Generally, increase in collector concentration resulted in increase in mineral recovery. However, flotation recoveries of columbite and pyrochlore at pH 8 were 3 and 5 percent lower with 250 mg per liter collector than with 25 mg per liter. Otherwise, increase in collector concentration increased recovery and broadened the pH range for complete recovery. Recovery data for ore minerals are given in table 7. Data plotted in figure 12 show the optimum pH range for ore mineral recovery to be pH 9 to 10. The effect of collector concentration on recovery of ore minerals is shown in figures 12 and 13.
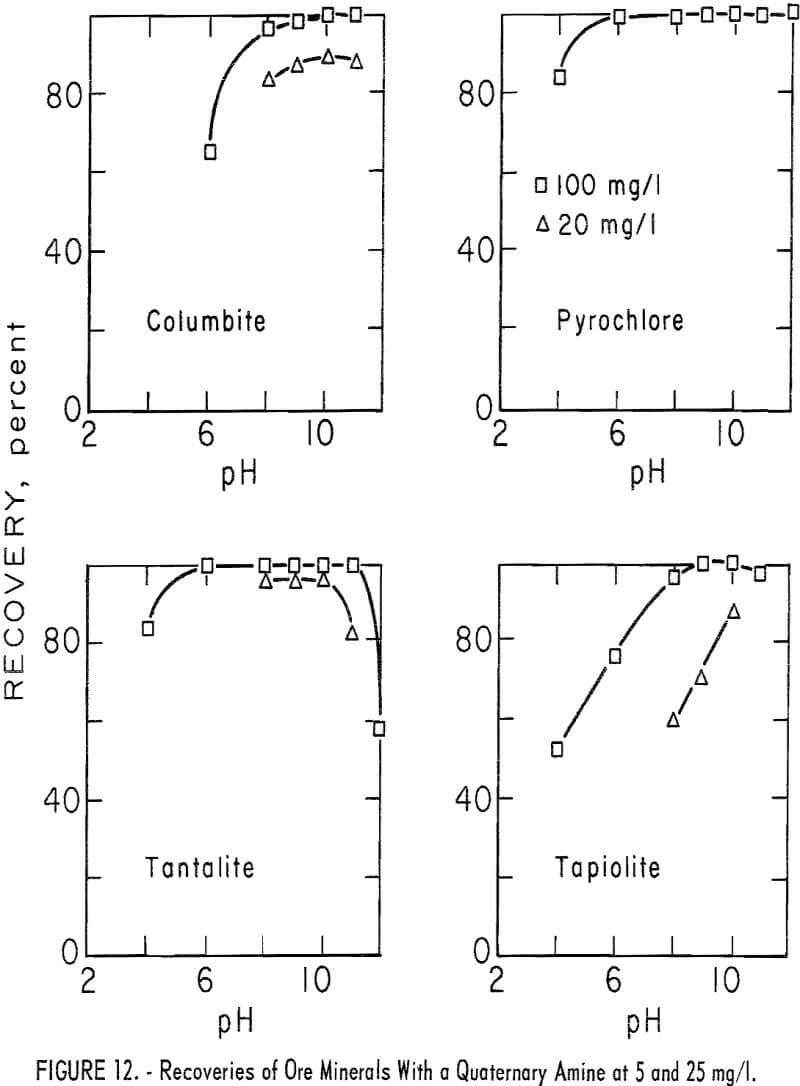
Quartz and microcline recoveries were equal to or higher than recoveries of ore minerals for a given collector-concentration level and at the same pH level. Calcite recoveries, however, were lower. At the 5-minute flotation time, calcite recoveries were below 25 percent in the pH range pH 6 to 11. In all tests calcite recovery was below 1 percent during the first minute of flotation.
Batch Flotation Studies
Batch flotation of natural ores was made to determine the effectiveness of specific collectors selected from contact-angle and microflotation studies.
The results of batch flotation of natural ores confirmed the collector characteristics predicted by microflotation testing. Successful separations of both pyrochlore and columbite from carbonate gangue minerals were made with either a primary or a quaternary amine collector; however, separations of pyrochlore from siliceous gangue were difficult.
Standard batch-scale laboratory equipment was used in preparation of test feed and in the rougher flotation step. For the cleaner flotation step, cells ranging in nominal capacity from 25 to 250 grams were used.
Two major columbium-bearing ores were studied: A pyrochlore ore from Oka, Quebec, Canada, and a columbite ore from Ravalli County, Mont. In each of these ores, the major gangue minerals were alkaline earth carbonates; however, each ore differed in overall character. The ore bodies from which the two major samples came are both complex and variable.
The pyrochlore ore was mill feed from the Oka operation of the St. Lawrence Columbium and Metals Co. The sample contained 0.54 percent Cb2O5, <0.01 percent Ta2O5, and 2.1 percent P2O5. An estimate of its mineralogical composition is given in table 8.
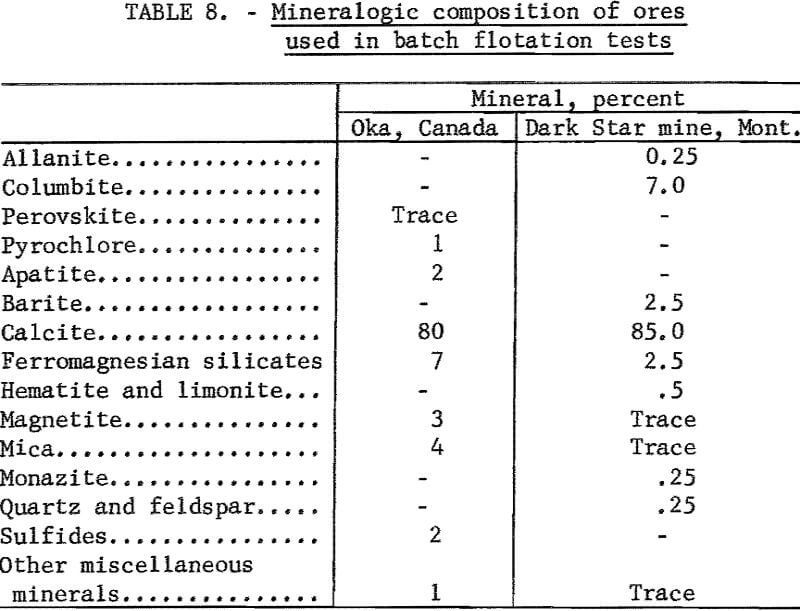
The columbite ore was from the Dark Star mine, Ravalli County, Mont. It contained 4.35 percent Cb2O5. A mineralogical analysis of this ore is given in table 8 and shows that columbite was the only columbium mineral present. Some locking of iron oxide minerals with quartz and calcite occurred. However, most of the columbite was liberated in the 65- by 100-mesh range.
Collectors used in batch testing were the same as those reported earlier in this paper, except for the use of a higher grade n-alkylbenzyl ammonium chloride containing a blend of C12, C14, and C16 alkyl groups.
Oka Pyrochlore Ore
The complex nature of the Oka ore and the limited selectivity of the collectors studied required -several separation steps to produce even a fair pyro-chlore concentrate. The goal of producing a commercial-grade (50 percent Cb2O5) pyrochlore concentrate was not reached; however, information was obtained which shows it should be possible to do so by the method studied if further recleaning steps were used. The separation methods included desliming, magnetic separation, tabling, and both anionic and cationic flotation.
After the preliminary separations of sulfides, slime, and magnetite, pyrochlore was floated and the bulk of the carbonate gangue rejected. In this rougher flotation step, cationic collectors were used. The collector used in most of the test work for this rougher step was n-alkyldimethylbenzyl ammonium chloride. A few tests were run with n-dodecylamine acetate. This primary amine was a strong collector for pyrochlore; however, under the conditions tested it was less selective than the quaternary amine. Results of rougher flotation with n-dodecylamine acetate are given in table 9. With n-alkyldimethylbenzyl ammonium chloride the range of rougher concentrate grade was from 1.0 to 2.4 percent Cb2O5 with recoveries from 60 to 80 percent of total Cb2O5.
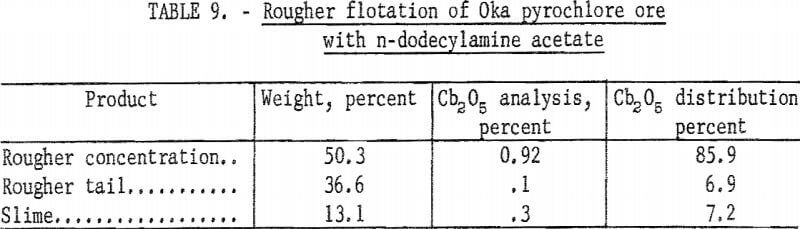
Typical reagent consumption for rougher flotation in pounds per ton was 0.5 n-alkyldimethyIbenzyl ammonium chloride, two each for NaF and for Na2CO3, and four for NaHCO3. For rougher flotation with dodecylamine acetate, consumption was 0.4 to 0.8 pound per ton.
Cleaning by cationic reflotation resulted in a concentrate grade of about 1.5 percent Cb2O5. Additional reflotation with cationic collector resulted in poor recoveries. However, with the anionic cleaning, the same or higher grade of cleaned concentrate (3.5 to 4.0 percent) could be attained with higher recoveries. In the second cleaner step, at pH 9.5, 0.3 pound per ton sodium heptadecyl sulfate was added, while in the third cleaner step, at pH 8.5, 0.09 pound per ton N-octadecyl disodium sulfosuccinamate was added.
The cleaned rougher concentrate was tabled to further concentrate pyrochlore and to remove the easy floating minerals such as mica, which made up a large fraction of the cleaned rougher concentrate. After tabling, enough residual n-alkyIbenzyl ammonium chloride remained on the minerals to make further flotation nonselective, if means to eliminate or nullify this collector action were not taken. The chief gangue minerals remaining in the pyrochlore concentrate after tabling were apatite and silicate minerals. In microflotation tests, sodium isooctyl phosphate was an excellent collector for pyrochlore at low pH levels. Microcline and quartz were collected to a lesser degree. However, when sodium isooctyl phosphate was used in cleaning the table concentrate, flotation selectivity was lower than predicted. Sodium isooctyl phosphate also tended to float apatite. The best results in cleaning the table concentrate were obtained by first heating it above 250° C to eliminate residual cationic collector, then floating apatite with sodium heptadecyl sulfate, followed by repeated cleanings with sodium isooctyl phosphate. A recovery of 36.4 percent of the Cb2O5 content was made at 44.4 percent Cb2O5 concentrate grade.
Visual examination of the flotation products showed each stage of the final cleaning step increased the grade of the concentrate. Cleaning was stopped on visual estimate of grade after observations of flotation conditions demonstrated that upgrading was continuous.
Attempts to clean the table concentrate with sodium oleate collector and hydrofluoric acid and/or starch as modifiers were not satisfactory.
Dark Star Ore
Flotation separation of the Dark Star columbite ore was less complex than that of the Oka ore as sulfides and micaceous minerals were not present. Calcite was the major gangue mineral. Lack of liberation between the gangue minerals quartz, calcite, and hematite and the relatively low collector selectivity between columbite and quartz and between columbite and hematitic minerals complicated the test procedure. Columbite was essentially liberated at 65 mesh; however, locking of calcite, quartz, and oxide iron minerals with each other was more intimate.
Flotation treatment of the Dark Star ore consisted of (1) stage grinding; (2) desliming; (3) rougher flotation at pH 9.0 to 9.5 with n-dodecylamine acetate; (4) reverse flotation with N-octadecyl disodium sulfosuccinamate for more rejection of carbonate minerals; (5) cleaning at pH 6.5 to 7.5 to reject hematitic gangue; and (6) multiple cleaning with HF at a low pH level to reject the remaining siliceous gangue.
Samples were stage-ground through 100 mesh and deslimed by sedimentation to remove 20-micron slime. The usual loss in desliming was approximately 10 percent of the total Cb2O5. It was determined by chemical and X-ray diffraction analysis that essentially all of the Cb2O5 loss was as columbite.
In the rougher float n-dodecylamine acetate collector in the amount of 1.5 pounds per ton of original feed or 1.75 pounds per ton of deslimed feed was used. Three-fourths of the total rougher collector requirement was added during conditioning at 30 to 50 percent pulp density. The remainder of the collector requirement was added stepwise after the dilution of pulp to normal flotation density. Flotation of the majority of the columbite was rapid. Coarser columbite floated less readily than finer sizes.
The rougher concentrate contained, in addition to columbite, fine slime, quartz, calcite, and minor minerals, which included hematite and hematite-coated grains of calcite and quartz. As a low pH level was required for the final cleaning step, removal of carbonate minerals was desirable. This was accomplished with wetting agents which acted as collectors for the slime and carbonate minerals in cleaning the rougher concentrate from the primary-amine float. Columbite that floated with the gangue minerals could be scavenged from the float products by repeated reflotation.
An effective collector for this reverse flotation cleaning was N-octadecyl disodium sulfosuccinamate. It was used as a 35 percent water base paste in amounts of 1.55 pounds per ton of original feed or 4.15 pounds per ton of rougher concentrate. The grade and mineralogical makeup of the scavenger concentrate (nonfloat fraction) was similar to that of the first cleaner concentrate, and it could be combined with it for the next cleaning step.
Iron minerals and iron-stained quartz and iron-stained calcite were present in this combined concentrate as well as residual N-octadecyl disodium sulfosuccinamate. Because of the presence of remaining carbonate minerals, separation at a pH level above 6.5 was desirable. The ferruginous gangue now floated in the pH range between 7 and 8, with addition of 0.4 pound of n-dodecylamine acetate per ton of cleaner concentrate. Selectivity in the final cleaning was improved by washing and repulping the nonfloat (columbite concentrate). Columbite floated well at pH 2.4 with about 1,5 pounds of dodecylamine and with about 5 pounds of 48 percent HF per ton of cleaner feed; however, multiple flotation was required.
Multiple flotation steps resulted in increased rejection of quartz from the columbite concentrate, but also, with each cleaner flotation step, columbite losses increased. Therefore, to continue improvement of concentrate grade and maintain reasonable recovery, several steps of scavenger flotation of the cleaner tails were necessary. Scavenger concentrates were recycled for recleaning.
From the results obtained and observation of the progress of cleaning, additional gangue could be rejected from columbite concentrate by further cleaning, and additional columbite would be recoverable by further scavenger flotation. Batch testing was carried only far enough to show satisfactory columbite flotation and cleaning.
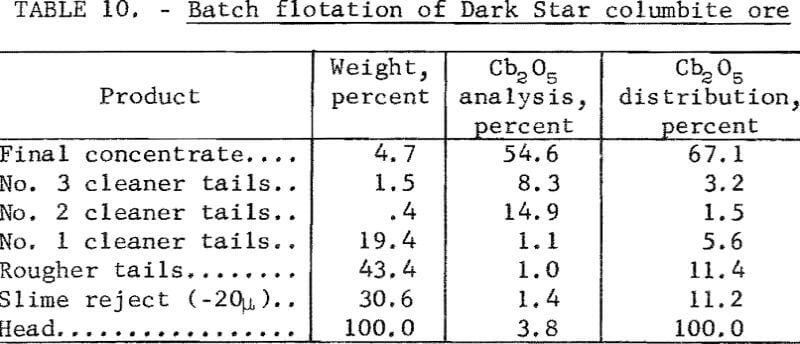
A metallurgical balance of one of the tests is given in table 10. The Cb2O5 content of the calculated head, in table 10, is lower than the analysis of the feed due to losses of concentrate during the multiple steps in final concentrate cleaning.
Conclusions
- Several collectors were identified by contact angle and microflotation studies which would float columbite and pyrochlore from carbonate gangue or silicate gangue. However, for complex ores, a more detailed study would be necessary for practical application.
- HF promotes the flotation of major columbium minerals.
- Modification of the free-bubble method by use of pressure induction of the test bubble resulted in improved reproducibility of observations of contact angles on nonsulfide minerals.
- Two wetting agents, N-octadecyl disodium sulfosuccinamate and sodium heptadecyl sulfate, were useful as auxiliary collectors in separations of apatite and carbonate minerals from pyrochlore and for separation of apatite, hematite, and carbonate minerals from columbite.
