Floatability of colemanite was determined using a 150-ml column cell (25 x 220 mm) with a fine fritt and magnetic stirrer. One gram of sample was conditioned in 150 ml of a solution containing SDS for 10 min. and floated for one min. using nitrogen gas at a flow rate of 50 cm³/min. The microflotation tests were performed by an electronically controlled apparatus designed and constructed in our laboratories.
Zeta potential measurements were conducted by means of Zeta Meter 3.0 equipped with a microprocessor unit. One gram of mineral was conditioned in 100 cc of distilled water or in the presence of surfactant for 10 min. The suspension was kept still for 5 min. and let the larger particles settle. Each data point is an average of approximately 10 measurements. Details of the experimental procedure is given elsewhere (Celik and Yasar, 1995).
Adsorption tests were conducted in 20 or 40 ml glass vials. Two grams of colemanite sample was mixed in 10 cc or its multiples with a solid to liquid ratio of 0.2. The vials were shaken for 2 h on a shaker and centrifuged for 15 min. The supernatant was analysed by a two phase titration technique originally applied to anionic surfactants using dimidium bromide and disulfine blue as indicators (Reid, 1967; Powers, 1970). This technique is based on the formation of a complex between an anionic surfactant (sodium dodecylsulfate) and a cationic reagent (DAH). This complex is soluble in chloroform and changes from blue to pink in the presence of indicators. The adsorption density was calculated by the following formula:
Γ =(Ci-Cr)v/S.1000……………………………………………………..(1)
where Ci and Cr represent the initial and residual surfactant concentrations in moles/l, S the amount of solid in grams, v the volume of the solution in ml, and Γ the adsorption density in mole/g.
Results and Discussion
Colemanite is a boron mineral containing Ca2+ ion in its lattice structure. The following overall reaction is obtained when colemanite reacts with CO2 in atmosphere ( Celik and Yasar, 1995; Alkan et al, 1991):
2CaO.3B2O3.5H2O + 4CO2 + 6H2O ⇔ 2Ca²+ + 6H3BO3+4HCO3-……………………………………….(2)
The concentration of Ca²+ in equilibrium with the colemanite suspensions was determined by a calcium ion selective electrode and found to be 5 x 10-³ M. Colemanite exhibits acid-base reactions in the vicinity of minimum solubility which corresponds to pH 9.3.
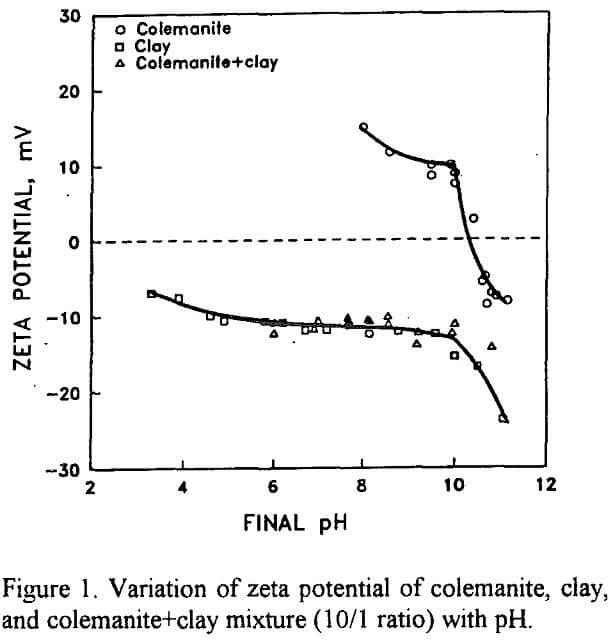
The variation of zeta potential of colemanite with pH in the absence and presence of clay is presented in Figure 1. The isoelectric point (iep) of colemanite is 10.5 in accord with the previous results (Celik and Yasar, 1995). The zeta potential of clay mineral shown in Figure 1 yields no iep in the entire pH range. The persistence of low negative zeta potentials up to pH 9.3 indicates the presence of minute amounts of borate ions that control the buffer pH of the suspension. The charge behavior observed in Figure 1 is typical of montmorillonite type clay minerals (Delgado et al, 1986). It is these negative charges that cause slime coating on colemanite. The zeta potential of colemanite-clay mixture shown in Figure 1 vividly illustrates the remarkable activity of clay mineral on colemanite. It appears that the clay coated colemanite mineral acts as if it were clay alone.