Table of Contents
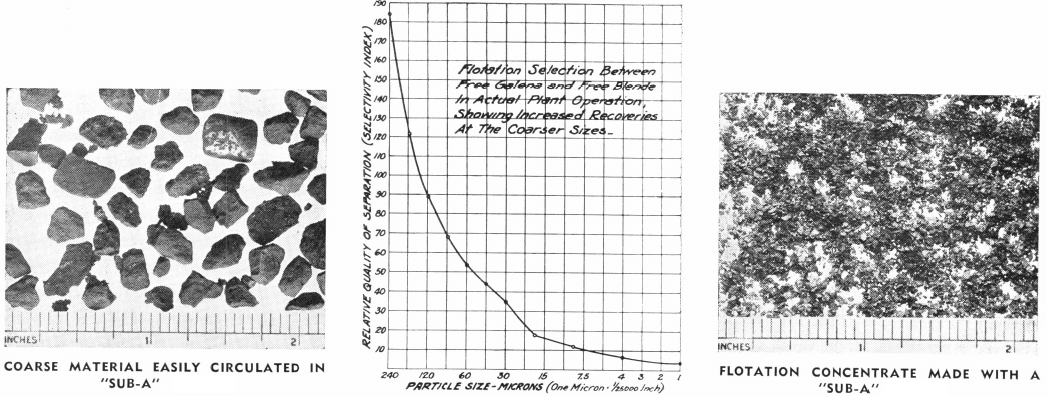
In The Days of gravity concentration, the art of ore dressing developed on the cardinal principle of saving the values at as coarse a size as possible. Flowsheets were designed which featured an alternation of crushing and concentrating operations. Thus an ore might be crushed to ½ inch, jigged to yield a finished concentrate and a middling; the latter being recrushed to ¼ inch, jigged a second time to yield an additional finished concentrate and a middling, the latter crushed again to, say, 1/20 inch and tabled to yield a finished concentrate and a middling; tailing and so forth.
The alternation of concentrating and crushing operations was carried out to save mineral as soon as it was liberated from the gangue and to avoid sliming for fine particles (that is, particles finer than 1/200 to 1/500 inch) as they are practically non-recoverable by gravity methods. In order to avoid the production of particles fine enough to be lost on tables, an extreme application of the principle of alternating—concentrating, screening, and crushing or grinding operations came in vogue, with the result that gravity concentrating plants became clumsy, elaborate, and expensive to build and operate. Like the dinosaurs of Mesozoic times, they became so clumsy that they disappeared when a competitor appeared which seemed to have none of their drawbacks.
In its early days flotation promised, not only the ability to treat slime, but also a great simplicity of flowsheets attributable to the fact that all that had to be done was to grind the ore to a fine pulp and then float it. Flotation was the powerful new tool to which all refractory ores would respond. Time, however, has shown that flotation is no cure-all, and there are poor as well as good ways of applying it.
Coarse VS Fine Flotation
It is a very natural assumption upon the part of many people that, the finer the particles are, the easier it is to float them.
This assumption has rested on two grounds:
- Finer particles in a given ore are more extensively liberated.
- Finer particles weigh less, therefore are more easily supported by flotation’s balloons—the air bubbles.
Although both of these statements are partially correct, there are other factors that must be considered in weighing the technical (as contrasted to the economic) advantages of fine grinding of flotation pulps. In the first place, finer particles settle slower through water. It has been shown by Prof. A. M. Gaudin that this slow settling causes fine particles to fail to meet the air bubbles, or to meet them with greater difficulty. This offsets the advantages which fine particles have of being carried more easily by the bubbles.
In the second place, fine particles cover more surface than coarse particles. This fact is, of course, of basic importance in the manufacture of paints, and in their application. Hence, since gas bubbles have in flotation a coat of particles generally not over one particle deep, more bubbles are required to float fine particles than to float the same quantity of mineral in the form of coarser particles. This technical objection has an economic corollary; finer gold flotation means higher power cost for flotation and decreased cell capacity.
In the third place, fine particles of gangue are more likely to overflow mechanically in the pulp carried over between bubbles, so that a high-grade concentrate is more difficult to obtain, and more cleaning operations may be required if a fine ground pulp is treated. The selection between floating and non-floating minerals is much reduced by excessive grinding, as may be seen on the graph above.
From a strictly economic standpoint, fine pulps are objectionable on the grounds that they cost more money to produce (high grinding cost), and more money to treat (high reagent consumption). The higher cost of grinding to produce fine pulps is well known, and is generally advanced by practical operators as the argument against fine grinding. The higher reagent cost to treat fine pulps may not be obvious at first sight, yet, when it is realized that the consumption of many reagents is in direct proportion to the surface of minerals in the pulp, this statement becomes evident.
To summarize, fine pulps have in their favor:
- Greater mineral liberation.
- More permanent attachment of particles to bubbles.
Coarse pulps on the other hand have in their favor the following arguments:
- Easier attachment of particles to bubbles.
- Increased flotation machine capacity.
- Decreased power consumption for flotation.
- Higher grade of concentrates due to less pollution of concentrates by mechanical overflow of fine particles of rejectable minerals.
- Lower grinding cost.
- Lower consumption of reagents of some types.
- Lower settling and filtering area required for concentrates.
Proper balance of these various items results in the best possible operation.
Application to Flowsheet Design
In this connection it is of particular, practical importance to consider the cases of selective flotation, and of collective flotation as applied to sulphide ores, and to precious metal ores, and to see whether it is possible to apply jointly the advantages of coarse flotation and those of fine flotation for greater operating efficiency.
In the case of many base metal sulphide ores such as lead, zinc, and iron it is customary to make several concentrates. Obviously, if the various metals are to be separated, the pulps must be ground fine enough to insure the liberation of the various minerals to a considerable extent. Since these sulphides are frequently inter-crystallized to an intimate degree, very fine grinding has to be practiced in many cases.
With most of the gold ores which are treated by flotation, the values are distributed among several sulphide telluride minerals. In these cases a collective flotation of the various sulphides has to be made. Since the aim here is not so much to make a clean concentrate as it is to reject most of the waste as nearly free of metal as possible, a much coarser grind is generally sufficient.
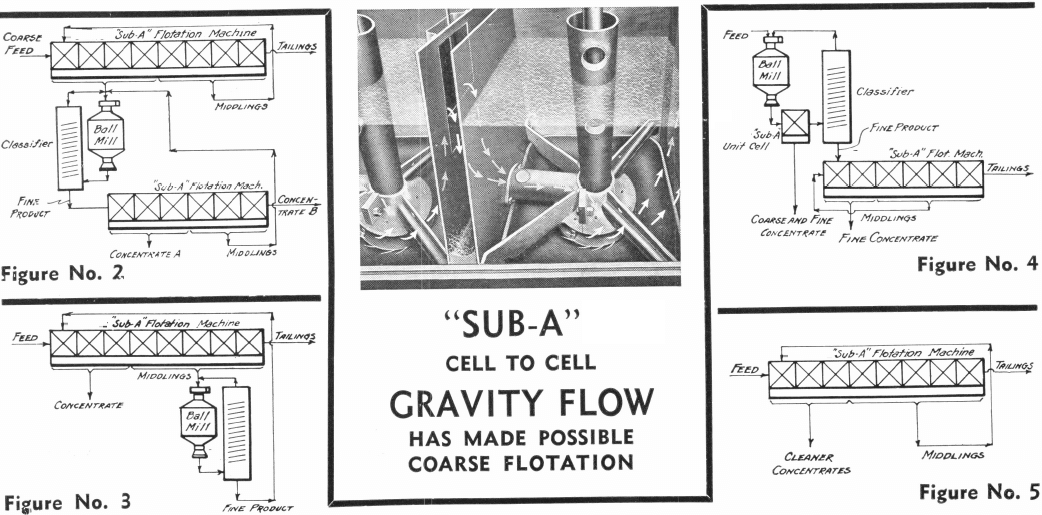
These extreme cases suggest that it may be possible to first make a collective or bulk float, then to regrind the concentrate and treat by selective flotation. See Figure 2. This practice allows the advantages of coarse flotation to apply to the whole ore, followed by the advantages of fine flotation to its valuable part. At the same time the disadvantages of fine flotation are restricted to a small part of the bulk of the ore, with the marked advantage of lower costs due to coarse flotation.
A variant of this particular practice, and one deserving of wider use, is that which consists of floating a concentrate and a middling from a coarse float, and in refloating the middling after regrinding as shown on Figure 3. The peculiar advantage of this method lies in the fact that mixed or locked particles respond but half-heartedly to flotation conditions to which free particles respond readily. They can, therefore, be collected in a middling product to which further grinding is applied. In many ways this is the ideal method. It causes grinding to be applied just where it should be instead of being applied blindly to a mixed ore pulp in which particles that are free are together with particles that require further grinding.
A most recently introduced variant of regrinding practice is that consisting in floating the “cream of the pulp” immediately from the discharge of the ball mill or rod mill before classification, as shown on Figure 4. The advantage of this method lies in the fact that the heavy—and generally valuable—minerals which tend to be ground finer because of their faster settling in classifiers and consequent return to grinding mills, are removed from the classifier-ball-mill circuit as soon as they are freed. This condition applies most emphatically to the treatment of gold ores. In several gold mills nearly 50% of the gold content of the ore is recovered in a higher grade concentrate by the introduction of a Unit Cell between the grinding mill and classifier.
facility with which coarse particles float and of the greater liberation of fine particles, are presented in the diagrammatic flowsheets (Figures 2, 3, 4). These flowsheets are to be contrasted with the flowsheet in Figure No. 5, which is characteristically not flexible.
In conclusion it will be apparent that the present trend in flotation is to float the mineral as soon as it is freed, pursuing it with grinding and concentrating devices until it reports in the concentrate as a substantially free particle. But the tendency is emphatically such as to make the chase as short as possible. After twenty years of flotation practice the art has returned to the cardinal gravity concentrating principle, with the difference that the dead-line is at about one twenty-thousandth of an inch instead of being at one two-hundredth of an inch.
Source: This article is a reproduction of an excerpt of “In the Public Domain” documents held in 911Metallurgy Corp’s private library.
