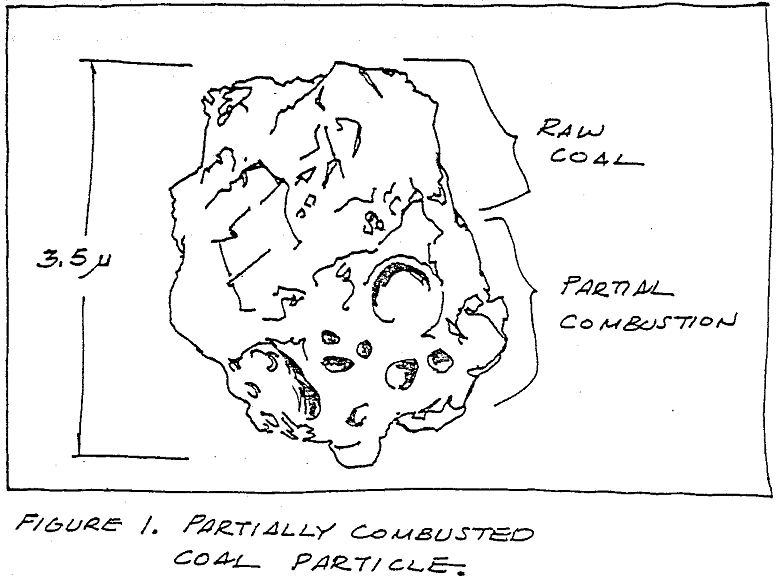
The combustion of pulverized coal is a century-old practice. Many people have investigated coal dust and the residual fly ash particles that remain after combustion. But the combustion process and fly ash formation mechanism are not understood. Working at our Maysville, Kentucky lime plant, the Dravo Lime Company Research team has collected samples of partially burned coal dust from the flame of a pulverized coal burner. Specimens were placed in a scanning electron microscope (SEM) equipped with an energy dispersive spectrometer (EDS). Microscopic examination of the particles, together with a large collection of micrographs of lime dust fused to fly ash particles, now make it possible to theorize as to how combustion advances through a piece of coal dust.
Sulfur content of the melted ash structure appears to be a function of the combined concentrations of calcium, potassium and sodium. Potassium and sodium are equally effective and together they must exceed 10% of the calcium concentration to maximize the sulfur content of the ash. To avoid a recirculating sulfur load in a lime kiln, it is preferable that sulfur be in the form of SO2 in the kiln gases. But there seems to be a quaternary eutectic of CaO, CaSO4, (K, Na)2SO4, (K, Na) (F, Cl) that is particularly effective in pulling SO2 from the gas into the ash melt. This phenomena highlights the fact that lime making is a thermochemical process where temperature, time and chemical reactions are interdependent.
The ideal combustion scenario starts with coal dust having a relatively narrow size distribution range. Being able to eliminate the large size particles (+ 100M) is more important than increasing the ratio of -325M to -200M particles. A burner system can be designed for a given mean size of coal particles but oversize particles will quickly degrade the performance of the design. The pulverized coal enters the kiln through a burner pipe which has almost any configuration other than round. Combustion on a macroscopic scale is a diffusion process where the oxidation reaction starts on the outside of the plume and advances radially inward.
As the coal particle leaves the burner pipe, it should encounter turbulent gas. Heat from the kiln gases and radiant heat from the kiln interior will warm the lighter molecular weight compounds in the particle to the ignition point. Turbulence will increase the rate of oxygen diffusion into the combustion region. Very little is actually known about the gas temperature profile in a kiln. The inlet conditions at the firing hood and the exhaust conditions at the product feed end of the kiln are readily measured. The refractory temperature in the firing zone is often 1400°C. T