The knowledge of aggregate size under various chemical and hydrodynamic conditions is very important for selective agglomeration, flotation and water clarification processes. Traditionally, aggregate size measurements are conducted using an ex-situ technique or in a diluted suspension. In either case, the measurement occurs in an environment that does not resemble the actual conditions under which the aggregates are formed.
Experimental
A run-of-mine Elkhorn No. 3 coal sample (United Fuels, Kentucky) and a Pittsburgh No. 8 coal sample (Consolidated Coal Company, Ohio) from a coarse coal jig product were collected for this investigation. Upon arrival, both samples were crushed and screened to acquire a 2-inch by 14-inch size fraction. This size fraction was cleaned in a magnetite bath having a specific gravity of 1.3.
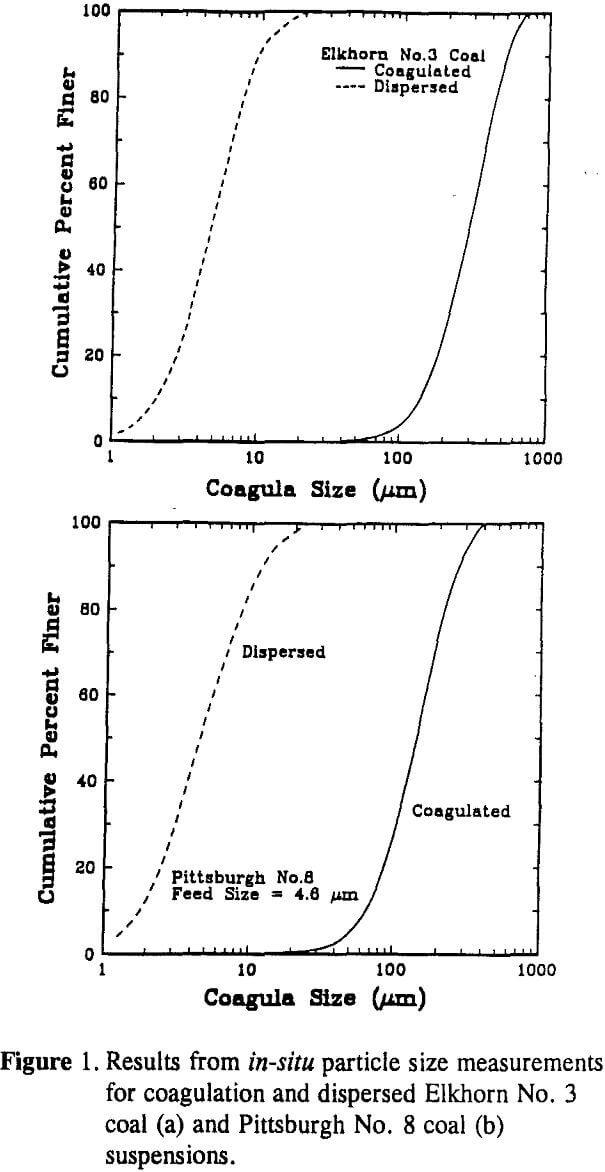
Coagula size distributions were measured in-situ using a Lasentec Model 100 particle size analyzer. The Lasentec particle size analyzer utilizes back-scattered laser light to infer the particle (aggregate) size. The width of the back-scattered pulses is monitored to predict the particle (aggregate) size distribution. Since the intensity of the back-scattered light does not contribute to the particle size analysis, size distributions can be obtained in suspensions having a high solids concentration.
The size fractions were analyzed using an Elzone 80-xy particle size analyzer to obtain the actual mean particle size (D50). From this data, an empirical relationship (which was found to be linear) was developed to relate the D50 obtained from the Lasentec analyzer to the actual D50 determined using the Elzone analyzer.
Coagulation Efficiency Measurements
Each experiment was conducted by suspending 10 grams of sample in a 500-ml KCl solution (10-³ M), and agitating it for 5 minutes at 750 rpm. After agitation, the suspension was allowed to settle for 3 minutes before siphoning the top 300 ml of supernatant. The solids in the supernatant were weighed after filtration and drying. The coagulation efficiency (Ec) was then calculated using the expression:
Ec = 100(Wt – Wf)/Wt
where Wt, is the initial weight of solids in the supernatant prior to coagulation and Wf is the weight after coagulation.
Results and Discussion
FThe larger D50 value obtained for the Elkhorn No. 3 coal suspension may be explained by a higher degree of surface hydrophobicity as indicated by water contact angle measurements (i.e., 67 versus 63) and lower surface potentials (i.e., -25 versus -32). A higher degree of surface hydrophobicity also provides a larger FB which decreases the breakage rate. Thus, the higher coagulation rate and lower breakage rate both contribute to the formation of larger coagula.
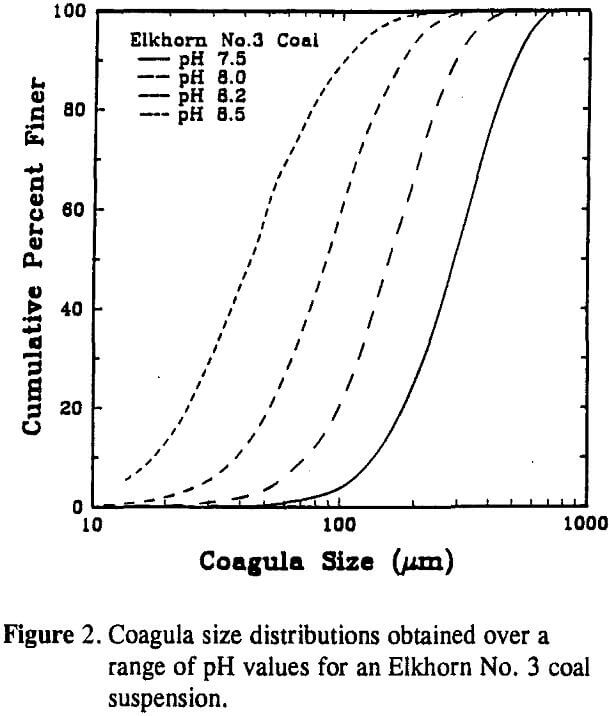
For the case of coal, surface potential can be changed by increasing or decreasing the suspension pH. To examine this effect, coagula size distributions were obtained for pH values between 7.5 and 8.5 using the Elkhorn No. 3 coal sample. The aggregate size measurements were performed while agitating the coal suspension at a rotational speed of 25 rpm. The size distributions indicate that the aggregate size decreased as the pH of the slurry was increased. This result was expected since the surface potential of the coal particles also increased with pH. By raising the pH from 7.5 to 8.2, the mean coagula size was reduced from 290 µm to 88 µm. A further increase in pH to 8.5 resulted in a decrease in the D50 to 45 µm.
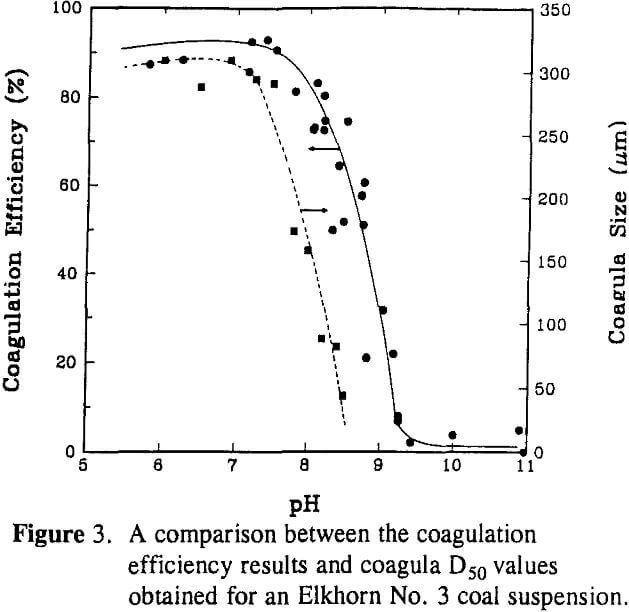
The aggregate size was found to decrease sharply from a D50 of approximately 300 µm to 30 µm when decreasing the solids concentration from 2.0% to 0.1 % by weight. Since population density has no direct influence on the breakage rate, the sharp decrease in the aggregate D50 size can be explained by a decrease in the aggregation rate with decreasing particle population.
The larger top sizes produced from the graphite suspension can be explained by the plate-like structure of the graphite particles. Since binding energy increases with an increase in contact area, the plate-to-plate contact between the graphite particles provides a substantially larger binding energy than the mixed-shaped particles associated with coal.

