Table of Contents
With zinc shaving precipitation the usual method of cleanup is to shut off the flow of solution and starting at the head compartment of a box to wash the shavings gently in the solution avoiding any action that would tend to break up the attenuated threads into short pieces. The zinc is then removed and after draining may be placed in a tub of water to protect it from oxidation by the atmosphere. A plug or cock is then opened at the bottom of the compartment and the solution, sludge, and short zinc allowed to run out into a launder which conveys it to the clean-up tank. The interior of the compartment is then carefully hosed out and cleansed with brushes or sponges, the outlet closed, and the screen-bottomed tray replaced. The long zinc is removed from the tub of water and carefully packed in, and the next compartment proceeded with in a similar manner, the shortage of zinc in the first being supplied by that removed from the second, and so on down the line. As soon as each compartment is cleaned and repacked the solution flow is opened and allowed to run long enough to cover the zinc and protect it from the air.
In some plants it is customary to replace the solution in the boxes with water before beginning the clean-up so as to avoid the troublesome effect of the cyanide upon the hands and arms of the workmen; when this is not done it is usual to supply rubber gloves for their use.
The clean-up tank may be six feet in diameter and four feet deep with arrangements for keeping the charge in agitation by means of stirring arms. At the top, on one side, is placed a large screen of from 20 to 60 mesh, which maybe supported on springs to assist in working the sludge through it. The latter after leaving the precipitation box runs to this screen where the coarse zinc is removed while the precious metal mud together with some fine zinc falls through into the tank, the operation being assisted by a stream of water or solution under pressure from a hose. For a large plant the screening of the zinc shorts is more conveniently done by running the contents of the zinc boxes to a revolving trommel provided with the desired mesh of wire cloth and a spray of clean water to wash the short zinc free from precipitate. The suction pipe of a small plunger pump is connected with the bottom of the tank and the product delivered into a filter press of the ordinary metallurgical type.
The broken zinc which is too big to pass the screen is usually returned to the zinc room to be utilized in one or other of the ways already described, but some metallurgists prefer to get rid of it either by dissolving it in acid, leaving a rich residue to be washed and filtered or by melting down in crucibles with a special flux. In some plants it is the custom to treat the whole of the precipitate with sulphuric acid before sending it to the filter press and in that case some or all of the “zinc shorts” may be allowed to enter the tank and share the acid treatment.
If the clean-up tank is to be used for acid treatment, it should be fitted with an adjustable hood and chimney to carry off the gases which are often poisonous and also with a decanter for removing the supernatant liquor after settlement.
When the precipitate is to be acid-treated it is advisable to displace the cyanide solution in the zinc boxes with water before beginning the clean-up so as to avoid the formation of hydrocyanic acid on the addition of the sulphuric acid. Water should be present to the amount of about 10 parts by weight to one of acid necessary, and the latter is added to the charge little by little, care being taken to see that the action is not sufficiently violent to cause it to boil over; the agitator meanwhile keeping the contents of the tank well stirred.
The acid sulphate of soda, NaHSO4, is sometimes used as a cheap substitute for sulphuric acid.
2NaHSO4 + Zn = Na2SO4 + ZnSO4 + H2.
When all action has ceased more water may be run in to fill the tank, and the charge may be allowed to settle. The clear liquor is decanted and more water added, agitated, settled and decanted as before. The thickened sludge is then pumped into the press and when the latter is filled the cakes are further washed by forcing water through them from cloth to cloth by means of the special washing ports. The Rand practice is to use hot water for this purpose for the more thorough removal of acid and sulphates. It is important that these be washed out as far as possible so as to minimize the formation of matte in the subsequent melting operations.
Whether acid treatment be used or not it is advisable to dry the cake down to about 10 to 15 per cent, moisture before fluxing and this may be done by spreading it on trays which are placed in a drying oven heated by steam or hot air. A small amount of moisture left in the cake tends to prevent loss by dusting when disintegrating and mixing with the flux. Many important plants do not dry the precipitate before fluxing except in so far as to allow compressed air to pass through the cake in the filter press for an hour or two before opening and discharging.
The early practice was to calcine the precipitate in a reverberatory or muffle furnace before melting so as to oxidize the zinc and cause it to enter the slag, but this procedure has now been almost entirely superseded by the acid process. In the treatment of silver and silver-gold ores the precipitate is usually of so high a grade that neither calcining nor acid treatment is necessary and direct melting will produce bullion from 850 to 950 fine.
Melting is usually done in graphite crucibles, whose size varies in different mills, ranging from No. 50 up to No. 400. The firing is done with coke or fuel oil; charcoal is sometimes used but has no sustaining power and requires continual replenishing, resulting in violent fluctuations of temperature. This effect may be remedied to some extent by using a mixture of coke and charcoal, and expenses may often be reduced in this way. In South Africa a reverberatory furnace capable of containing a dozen crucibles is sometimes used. In recent times the oil fired tilting furnace to hold one large crucible of No. 300 or 400 size has found considerable favor.
At the Nipissing Mill, the bullion from all sources is melted down in an oil-fired reverberatory. After the slag has been removed the metal is refined by playing a blast of air over the surface of the molten bath of metal by which means silver of 998 to 999 fineness is obtained.
The Nipissing type of furnace has recently found favor in Mexico and in many large plants has entirely displaced crucible melting, with a considerable saving on account of crucibles, fuel and labor. The precipitate cakes from the presses without any preliminary drying are placed on trays and covered with about 5% of flux, usually composed of borax and bottle glass and charged into the furnace at intervals until the desired amount of silver has been accumulated. The Nipissing refining-procedure is however, omitted, and as soon as the charge is fused, it is tapped out into shipping-bar moulds, the slag following at the end of the pour. About 70 shipping bars, each 35 kilos in weight, are produced at each fusion. When melting about 8000 kilos of fine bullion a month, the furnace bottom will last for 6 to 8 months before it has to be replaced.
Flux Recipe
The fluxes and their recipes that are ordinarily used will vary with the composition of the precipitate and in order to determine the best mixture a number of small fusions may be made with varying proportions and the results noted. Borax is the most generally useful reagent but if used in large quantity, it makes the fusion rather expensive. Its place may be taken to some extent by carbonate of soda and sand, utilizing the tendency of bases to form fusible double silicates. For this purpose only sufficient soda should be used to form the second base in the double silicate. When the solutions are not properly filtered before precipitation there may be a considerable amount of silica in the precipitate and it may not be necessary to add any sand, but such a state of things usually implies bad working conditions. A little fluor-spar is sometimes used to flux difficultly fusible substances. For soda most text books recommend the bicarbonate though for what reason it is difficult to see, and the writer prefers the normal carbonate in the form of commercial soda ash. Sodium bicarbonate evolves a large volume of carbonic acid gas at a temperature below that of sintering of the charge, and if the precipitate is too dry, a considerable loss by dusting may occur; moreover, soda ash is less bulky and also cheaper per unit of fluxing power.
Julian and Smart give the following as a typical flux recipe mixture.
Precipitate…………………………………………………………………………100 parts
Bicarbonate of soda…………………………………………………………….40 parts
Borax………………………………………………………………………………….40 parts
Sand……………………………………………………………………………………15 parts
Park gives,
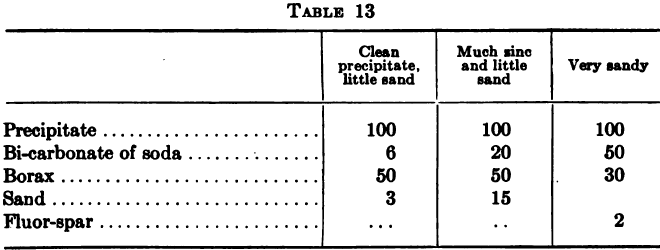
If sodium carbonate had been used instead of the bicarbonate only about 2/3 of the above amounts of soda would have been needed.
By adding an oxidizing agent to the flux it is often possible to raise the fineness of the bullion by oxidizing some of the base metal and throwing it into the slag. Nitre is sometimes used for this purpose but it has a low efficiency owing to the rapidity of its reaction and E. H. Johnson and W. A. Caldecott have substituted manganese dioxide, and suggest the following mixtures:
Precipitate…………………………………………………………………………..100 parts
Fused borax…………………………………………………………………………20 to 35 parts
Manganese dioxide……………………………………………………………….20 to 40 parts
Sand…………………………………………………………………………………..15 to 40 parts
They state that manganese dioxide has a tendency to carry silver into the slag, so if much silver is present, it should be used cautiously or not at all.
When an oxidizing flux is used the operation should be carried out in crucibles with removable or permanent fire-clay linings, as graphite would tend to counteract the effect of the oxidizing agent.
To one accustomed to the high-grade precipitates produced in the silver mills of Mexico the proportions of flux previously mentioned will seem excessive, and in such cases the quantity may be considerably reduced. A typical flux mixture for these
rich silver precipitates is that used by Walter Neal at the Dos Estrellas mine in Mexico.
Precipitate……………………………………………………………..100 parts
Borax…………………………………………………………………….15 parts
Bicarbonate of soda………………………………………………..8 parts
Sand……………………………………………………………………..4 parts
Scrap wrought iron in excess
The iron is used to remove the silver from the matte which is so often produced in melting these precipitates even when no acid treatment is given. The same writer, in cleaning-up, makes two short-zinc products. He first screens his material with a 20- mesh sieve and afterward with a 60-mesh. The zinc remaining on 20 he returns to the boxes and that passing 20 but remaining on 60 he melts separately with a special flux:
Short zinc…………………………………………………………………………….100 parts
Borax……………………………………………………………………………………40 parts
Soda……………………………………………………………………………………..20 parts
Sand……………………………………………………………………………………..10 parts
Lime……………………………………………………………………………………..5 parts
The resulting metal, which contains 20% of zinc, is added to the high-grade bullion when the latter is remelted for running into bars.
A flux the writer has often found useful for clean silver precipitates consists of
Precipitate……………………………………………………………………..100 parts
Borax………………………………………………………………………………15 parts
Sand…………………………………………………………………………………8 parts
Soda (Na2CO3)…………………………………………………………………..8 parts
Preparation of The Charge for Melting
Opinions vary as to the degree of mixture necessary. Some metallurgists break up the lumps of precipitate and pass the whole through a fine sieve, varying from ¼ in. to 20 mesh, before or during the addition of the fluxes, making a thorough mixture before charging into the crucibles, while others, avoiding breaking the lumps as far as possible, spread the precipitate in a layer on the fluxing table, sprinkle the fluxes evenly on top and shovel at once into the pot. The latter method has the advantage of reducing the dust loss to a minimum, but on the other hand is probably more suitable for a pure high-grade precipitate than for a base and dirty one, and the choice of procedure must be determined by the circumstances of each mill.
Briquetting has much to recommend it in the avoidance of accidental spilling of product outside the edges of the pot and minimizing of dust losses in charging, especially where it is the custom to add fresh batches of the material on top of a charge already partly fused, but it is expensive and also conducive to losses due to the additional handling necessary.
Charging
The practice in some mills is to charge a crucible only at the beginning of each fusion and to pour it as soon as ebullition ceases. It is a wise precaution against dust loss but is less efficient in fuel, time and labor, and its adoption should be determined by the value of the precipitate per unit of weight. To avoid spilling when charging, the fluxed material is sometimes put into stout paper bags holding from one to two pounds, which can be placed in the crucible with a pair of tongs.
Pouring
The most usual procedure is to pour the fused charge, slag and all, into a conical mould, and when cool to knock off the bottom and put aside for remelting into a shipping bar. At the Butters Divisadero Company’s mill, however, G. H. Clevenger some years ago introduced the practice of carefully tilting the crucible to decant the bulk of the slag into a conical mould, stopping when about an inch only of slag remained on top of the metal: a little dry sand was then thrown in to cool and thicken the slag, which was raked off with a small scraper, the pot being kept tilted so as to bring the level of the molten metal almost to the lip. The clean bullion was poured direct into the shipping-bar mould and a remelting avoided, resulting in a marked saving in fuel and labor. The first batch of slag removed was low grade and free from metallics, but the thickened slag had to be remelted and the thickening agent, being clean quartz, instead of bone ash, was easily fluxed with a little soda. The resulting bullion averaged from 900 to 950 fine in gold and silver.
Sampling
There are various methods in use for sampling the bullion bars for assay. Probably the most common is that of chipping off two diagonally opposite corners of the bar and averaging the resulting assays. Sometimes, however, the difference between them is so great as to throw doubts on the accuracy of both and in that case it will often be possible to obtain a better sample by stirring the molten metal and dipping out a little in a small well-heated ladle just before pouring. This sample may be granulated by pouring it while molten onto a sloping board immersed in a bucket of water, or better still, by pouring into a conical assay mould and when cold drilling a hole right through the centre of the button and assaying the borings.
The by-products of the melt consist of slag, sometimes of matte, and when coke or charcoal is used for firing, of ashes.
When the slag contains visible metallics it is usually ground up and concentrated either in a sluice box, a cradle, or on a Wilfley table and the tailings shipped to a smelter or thrown into the mill bins. The latter course has in some cases been known to complicate the cyanide treatment of the ore and should be adopted with caution.
The matte may be either shipped to a smelter or treated at the mill. There are various ways of accomplishing the latter. One is to melt the matte in a crucible with metallic iron, forming iron sulphide and throwing down the precious metal. Another is to blow air through the molten mass. A. E. Drucker recommends the following: the matte is broken fine in a rock breaker and alternate layers of borax, matte, and broken cyanide are charged into a No. 60 crucible until it is nearly full, a layer of borax being added as cover. The charge is fused down in the furnace at a white heat until tranquil. The slag will be thick, and is removed with a skimmer, the resulting metal being then poured into a mould, and the slag remelted with a subsequent charge. R. C. Kline, in a private communication to the writer, states that in some cases if the matte be ground to pass 200 mesh and treated with strong cyanide solution and a lead compound, a recovery of 99 to 100% of the assay content may be made.
Furnace ashes nearly always contain some precious metal and should be roughly crushed and run over a sluice box or otherwise concentrated. They should never be put into the mill to be treated with the ore on account of the presence of unconsumed carbon, which would tend to absorb gold from the cyanide solution.
The Tavener Process
