Table of Contents
The mining and mineral industries generate large amounts of fine particle slurries which are presently dewatered by impoundment, thickening, filtration or flocculation. Flocculation is a primary unit process that plays a major role in solid-liquid separation throughout the processing industry and waste water treatment facilities. The flocculation is carried out by agglomerating fine particles into flocs using high molecular weight polymers of positive, negative or non-ionic charge. It has been observed, in the laboratory and field tests conducted at TURC, that sometimes flocs fall apart if left in the supernate for a short period. During field tests, clay slurries have been encountered that could have been dewatered if rapid floc deterioration had not occurred.
It is generally accepted that the flocculation of clay-polymer systems depends primarily upon collision, adsorption, polymer re-configuration, bridging, and minimum re-dispersion. When the polymer is added to a slurry, it comes in contact with the clay surface due to agitation and forms bonds with the active sites. Theng (1979), has reported that non-ionic polymers tend to adopt a random coil conformation when in solution. After adsorption of the polymer, the tails and loops of polymer coil extend into solution and can bridge with other particles. However, with time, the coils spread out over the clay surface, Greenland (1964). The spreading of polymer stops the bridging process by eliminating almost all of the loops and tails of the polymer chain thus stopping the floc growth. However, if the spreading of polymer is stopped and the number of loops and tails of polymer coil is increased then the efficiency of the flocculation process also increases. The flocculation of clay slurries is a very complicated process and instead of following all of the above mentioned steps in succession they may be going on simultaneously.
Moudgil (1988), reported that the binding strength of clay-PEO is only 4.6 dynes/cm² which is a weak bond compared to other polymers like polyacrylamides. It has been shown in the literature that the adsorption of PEO on the clay surface is mainly due to hydrogen bonding, Webb (1986) and Somasundaran (1982). Because of the weak hydrogen bond, loops and tails of PEO coil tend to move around by breaking and reforming bonds until they spread over the clay particle surface. Since there is an increasing number of bonds as the polymer spreads over the clay surface, the clay-PEO flocs start breaking immediately after mixing and addition of polymer is stopped. With time, more surface and reaction sites are covered by PEO thus decreasing the number of loops and tails and increasing the floc decay rate.
For this study, the rate of floc decay was measured by recording the volume of PEO required to bring the floc back to its original structure. Experiments were also conducted to determine the effect of NaF addition on floc degeneration.
Materials and Procedures
For this investigation, experiments were conducted on a kaolin clay slurry prepared in the laboratory from dry samples which were obtained from the Thiele Kaolin Co. in Georgia. The slurry was prepared by mixing dry powder with water and was allowed to equilibrate for at least 6 h before it was used. The concentration of kaolin slurry used was 2 pct by weight and had a pH of 7.9. Polyethylene oxide (PEO), a nonionic high molecular weight polymer, was used as a flocculant. The stock solution of PEO, 0.25 pct concentration, was prepared by following the standard procedure used in this laboratory, Scheiner (1985). For post dewatering floc decay tests, four different concentrations (0.01, 0.025, 0.05, 0.075 wt pct) of PEO were used.
The testing procedure generally followed the standard PEO procedure developed and previously used in this laboratory, Scheiner (1985). Batch tests were conducted by adding 100 mL of 2 pct kaolin slurry into a 400 mL beaker and stirring continuously with a magnetic stirring bar at 500 rpm. An automatic titrator was used to add the polymer to the slurry because of its accuracy and control of the rate of polymer addition. The polymer was added at the rate of one drop per 2 seconds until flocs formed and agglomerated, moving in the beaker as a coherent mass. The endpoint was carefully measured by observing the floc growth and size for each concentration of PEO. The volume of PEO used was recorded for the computation of polymer dosage.
For each series, six beakers of slurry were used and immediately after completion of the primary flocculation test, the timer was started to record time. Each beaker was treated with additional PEO, at different time intervals, to bring the flocs back to the original structure at time zero. In this paper, PEO volume used during the primary test is indicted by time zero. After completion of the first series of flocculation tests, the flocs were left in the supernate and stirring was stopped. The flocculated material started to decay immediately after completion of the primary test. The disintegrated samples were again flocculated to bring the flocs back to dewaterability by following the aforementioned procedure and the PEO volume, used during the secondary test, was again recorded. The secondary re-flocculation tests on degraded clay flocs were conducted at time intervals of 2, 4, 6, 8, 10 and 12 min. Both primary and secondary flocculation experiments were repeated three times and the data was factor analyzed, to take the errors out, before it was used, Stanley (1988).
Preliminary experiments were also conducted to determine the effect of fluoride ion addition on the decay of flocs. For these tests, various dosages of sodium fluoride were added to the slurry and stirred for 1 min before primary dewatering tests began. After completion of the primary dewatering tests, the same dewatering procedure was used to follow the decay of fluoride-treated flocs.
Theory
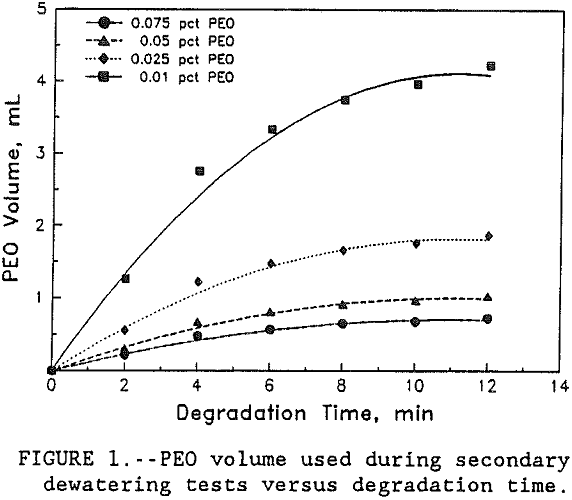
In this study, PEO volume, used to bring flocs back to the original size, and the time of floc degradation was recorded during secondary flocculation tests. Figure 1 shows the graph between degradation time and the polymer volume used. Based on the data obtained, figure 1, a kinetic study of floc breakage in the supernate, in which PEO was adsorbed through segments on the clay surface was conducted using the standard first order kinetic equation, Laidler (1950):
da/dt = -k(am-a)……………………………………………………..(1)
where,
a = Floc breakage measured by the amount of polymer used at time t, mol/l,
am = Maximum floc breakage measured by the amount of polymer used at time t>12 min, mol/l,
t = Time, min,
k = Rate constant for floc adsorption, sec-¹
This equation is first order with respect to “a” because am is constant. Hence, the equation is first order with respect to the polymer used for degradation at time t which is directly related to the amount of floc degradation. Therefore, it is assumed that the aforementioned equation is first order with respect to the degradation of floc. Integration of equation 1 gives:
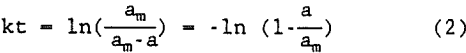
A plot of -ln[l-(a/am)] versus t gives the rate constant k for the degradation of flocs in supernate.
To use the aforementioned equation for floc degradation studies, the following assumptions were made:
- The final polymer concentration, in the supernate, following the adsorption process, is only a small fraction of the initial polymer concentration, (Jankovics 1965).
- The initial amount of polymer used was calculated from the primary dewatering tests and it was assumed that the amount of polymer added during both flocculation series is the amount of PEO adsorbed.
- After the primary dewatering tests, the extent of floc decay was calculated indirectly by measuring the amount of polymer adsorbed with respect to time to bring the flocs back to the initial dewatered level floc size.
Results and Discussion
A computer program developed at the Bureau of Mines’ Tuscaloosa Research Center was used for factor analysis. This program uses either a covariance or correlation matrix as the starting point for the factor analysis and is used for the error removal from the experimental data, Malinowski (1980). For this study, a 4 by 6 matrix was formed using PEO concentration as columns and the time of floc deterioration during secondary tests as rows. Since there were 4 columns in the matrix, factor analysis generated 4 factors. From these, only one underlying factor had a physically significant meaning. The data was factor analyzed to remove the experimental errors before it was used in the calculations, Stanley (1988).
For this study, flocs were left in the supernate after primary dewatering tests, as mentioned in the materials and procedure section. Therefore, PEO started breaking and forming new bonds until each molecule reached a steady state. For non-ionic polymers such as PEO, the steady state was attained by spreading over the particle surface and making these molecules unavailable for further bridging. Because of this process, the polymer was no longer coiled; therefore, the polymer-polymer frictional interaction decreased and clay aggregates started falling apart. The rate of this decay was measured by recording the volume of PEO addition needed, during the secondary tests, to bring the flocs back to the original structure.
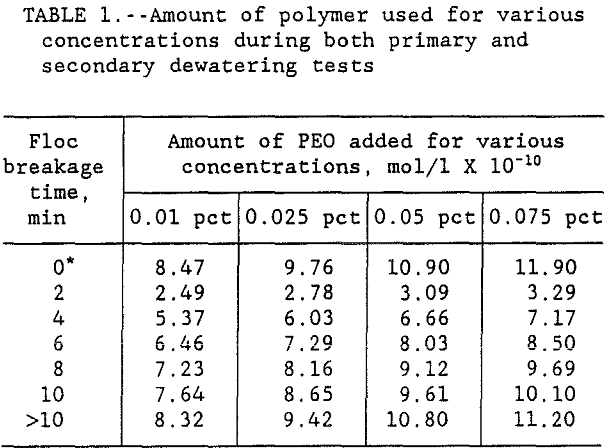
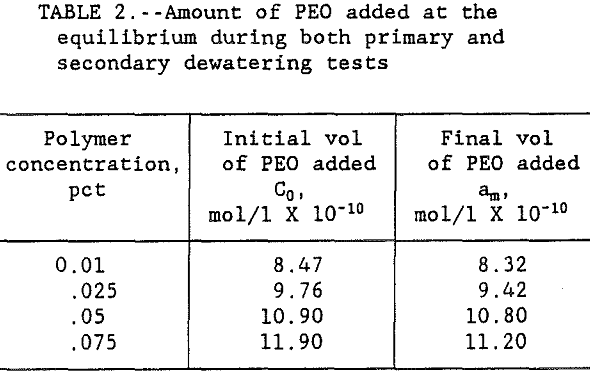
The polymer volume used in both primary and secondary flocculation tests was converted to moles of polymer per liter of solution. The results in table 1 show that the amount of PEO added to replace the polymer lost by spreading increased with increasing PEO concentration. Results also show that as the time flocs were left in the supernate increased from 0 to >10 min, the amount of PEO added increased asymptotically to the initial dosage used in the primary tests. These results indicate that the floc breakage increased with the increase in time until the amount of PEO required for re-flocculation asymptotically approached the pre-primary test level dispersion. Table 2 shows the data on PEO addition during both primary, C0, and secondary, am, dewatering tests.
A graph of time versus -ln[ l-(a/am) ] was plotted for each concentration of PEO. Since all four of the curves produced very similar slope, only the 0.01 pct PEO concentration curve is shown in figure 2. The data in table 3 shows that the rate constant for floc deterioration in supernate, k, decreased from 0.25 to 0.23 sec-¹ with the increase in PEO concentration. However, for all practical purposes, these numbers are constant and the small variations may be caused by the
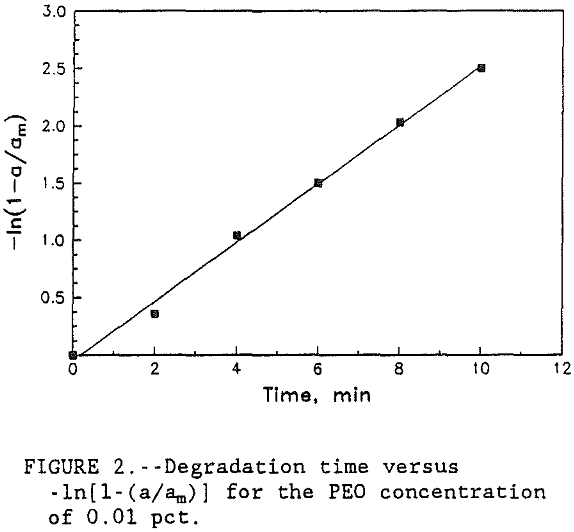
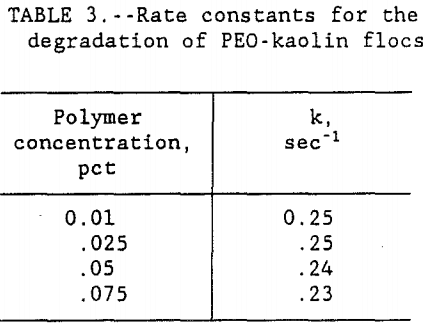
experimental errors. Therefore, the results show that the rate of floc degradation of the PEO-kaolin system is of first order and reaction rate constant, k, is 0.25 sec-¹. Results also show that the rate of floc degradation is dependent on polymer concentration whereas the rate constant is independent of concentration. Although the rate constant for floc degradation is slightly higher at low polymer concentrations, it is still more efficient to use the lower concentration because the amount of PEO required to rejuvenate the degraded flocs is less for the lower concentration of polymer.
Previous research has indicated that the addition of NaF retarded floc degradation of the clay-PEO system. A series of experiments was conducted to determine the effect of NaF on the PEO-kaolin system. Preliminary results show that when the NaF dosage was increased from 0 to 15 lb/ton the PEO volume adsorbed in the secondary tests decreased from 3.97 to 0.63 mL, indicating that as the number of fluoride ions increased, the floc breakage decreased. Proposed explanations of this mechanism are that the fluoride ion reacts with available sites on the clay particles by hydrogen bonding or that it forms a complex with aluminum on the edges of clay particles. The decrease in the number of bonding sites prevented the PEO from spreading on the clay particle surface. Because of this, PEO is stopped from going to its flattened steady state configuration on the clay surface, resulting in more loops and tails thus maintaining the floc stability in the supernate. This was a preliminary investigation on this subject and a detailed study is currently being conducted using a Zeta meter, SEM, and FTIR spectroscopy.
Conclusions
In this study, the amount of PEO required to bring back the floc to its original structure was used as the measure of floc disintegration in the supernate after primary dewatering tests. The results show that the amount of polymer added, to replace the PEO lost by spreading, decreased with the decrease in PEO concentration. For this clay-polymer system, the data in figure 1 showed that for all four concentrations, floc breakage as judged by the polymer adsorption followed first order kinetics. Although it was found that the rate constant for floc degradation, k, decreased from 0.25 to 0.23 sec-¹ with the increase in PEO concentration from 0.01 to 0.075, the decrease was very small and for all practical purposes it was constant. Therefore, this study has shown that the rate of floc degradation of PEO-kaolin system is of first order and the rate constant is independent of polymer concentration. Preliminary work done on the addition of fluoride ion shows that with the increase in its dosage, the floc degeneration decreased. The proposed mechanism for this observation is that fluoride ion interacts with the clay surface and stops the PEO from spreading.
