Table of Contents
Under its mission to effect pollution abatement, the Bureau of Mines began intensified research seeking means for economically disposing of the large amounts of Florida phosphatic clay wastes and reclaiming the mined land. The clays, which are discharged from the processing plants at about 3 to 5 pct solids , have colloidal characteristics and are difficult and slow to dewater by natural settling. The quantity of water retained and stored with them is so great that impoundment in above ground, dammed storage areas is required. Boyle has estimated that one processing plant produced 4 million tons of phosphatic clay wastes per year, and Vasan has estimated that about 2 billion tons of clay wastes are stored behind impoundment dams. The stored suspensions represent a serious potential danger for pollution to the land and aquatic environments of the area because of possible dam failure.
The Bureau of Mines is developing a method for dewatering the suspensions of clay wastes using polythylene oxide (PEO) as a flocculant. Polyethylene oxide can act as a bridge between colloidal particles causing agglomeration that results in rapid separation of clays from water. Once the water is released from the agglomerates, it is removed immediately by mechanical means. The dewatering technique allows for disposal of flocculated clay wastes, for reuse of water now lost with the clays, and for reclamation of mined land.
The fate of the PEO contained in the consolidated clay wastes could be important because environmentally unacceptable degradation products are thought by some to be possible. Polyethylene oxide is prepared by catalytic polymerization of ethylene oxide gas, a chemical designated as a hazardous substance to humans. Polymerization results in a rupture of the highly strained, very reactive ethylene oxide ring structure in order to form a much more stable and less reactive polymer chain structure. Although the possibility that ethylene oxide could reform is highly unlikely based on thermodynamic data, supportive experimental evidence that gaseous ethylene oxide is not present is necessary prior to large-scale testing of the PEO flocculation dewatering technique. This report describes a study to determine whether or not polyethylene oxide degrades to ethylene oxide gas as a result of contact with various clay waste environments. The report also includes a comprehensive literature survey on the properties of both PEO and ethylene oxide and a discussion of possible degradation products of the polymer.
The Bureau of Mines, U.S. Department of the Interior, has developed a novel method of flocculation dewatering of phosphatic clay wastes using polyethylene oxide as the flocculant. Research was conducted to determine whether ethylene oxide gas was present in the air in the vicinity of disposed waste materials which had been flocculated with polyethylene oxide. Samples of clay waste materials containing polyethylene oxide were prepared in stoppered glass bottles in simulated disposal environments. Gaseous samples, removed over a 75-day period using an airtight syringe, were injected into a gas chromatograph that was capable of separating ethylene oxide from air. The presence of ethylene oxide gas was not detected in any sample. To determine possible degradation products of polyethylene oxide, the properties and reactions of ethylene oxide and its polymers were reviewed. Based upon the literature survey and experimental study, it was concluded that adverse environmental effects were not likely to result from the use of polyethylene oxide for flocculating phosphatic clay waste products.
Properties of Ethylene Oxide
Ethylene oxide, also referred to as epoxyethane, oxirane, or oxacyclopropane, has the chemical formula C2H4O with the epoxy ring structure shown in figure 1.
Ethylene oxide is the simplest cyclic ether. 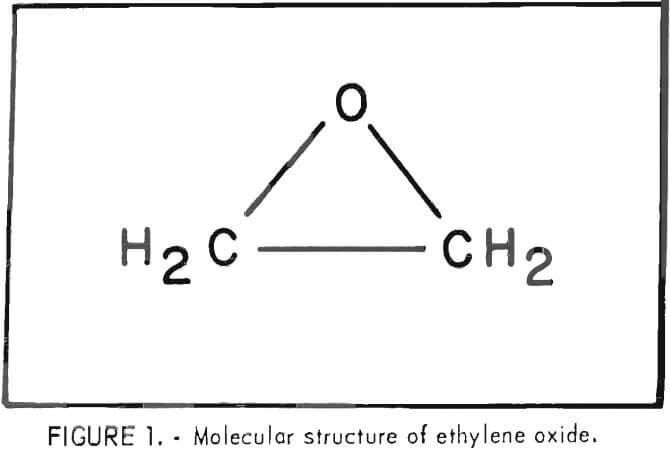 It is prepared commercially by direct combination of ethylene and oxygen at elevated temperatures in the presence of silver catalysts. It also can be obtained from ethylene cholorohydrin by dehydrochlorination with soda lime. The thermodynamic properties of ethylene oxide and equilibrium constants for its formation and hydration reactions are tabulated in the literature.
It is prepared commercially by direct combination of ethylene and oxygen at elevated temperatures in the presence of silver catalysts. It also can be obtained from ethylene cholorohydrin by dehydrochlorination with soda lime. The thermodynamic properties of ethylene oxide and equilibrium constants for its formation and hydration reactions are tabulated in the literature.
Ethylene oxide is a highly reactive species due to the large strain energy of the three-membered epoxy ring. The strain required to form the three-membered ring results in a C-C bond 0.07A shorter than a normal C-C bond. The bond angles reported in the literature are 61° 41.2′ for C-O-C and 59° 9.4′ for each O-C-C. Compared with the normal tetrahedral carbon angle of 109.5°, or the C-O-C angle of 110° for open-chain ethers, the angles are indeed considerably smaller and quite strained in the opoxy ring. Due to the approximately 60° angles, there is less orbital overlap, which results in weaker bonds and a less stable, more reactive molecule. Ethylene oxide is important as an industrial chemical because of its high reactivity due to the ease of opening of the very strained ring.
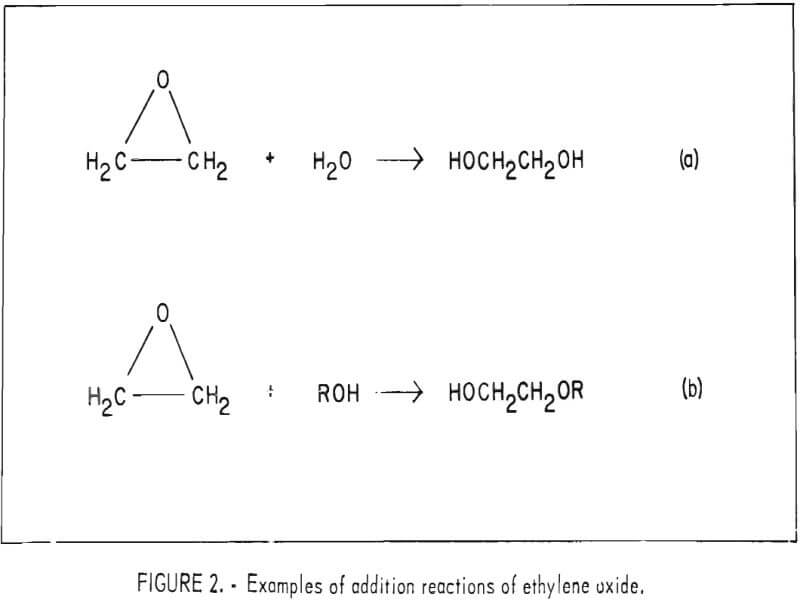
Additional reactions are common between ethylene oxide and compounds containing an active hydrogen atom, such as amines, alcohols, and phenols. For example, ethylene oxide reacts with water to form glycol or polyglycol, and with alcohols forming hydroxy ethers and monoethers of polyglycol as shown in figure 2a and 2b, respectively.
Ethylene oxide is a colorless, flammable gas. It has a vapor pressure of 7.3 psig at 20.1° C. It is widely used as a fumigant, fungicide, and sterlizing agent, subject to restrictions by regulatory agencies. The Occupational Safety and Health Administration (OSHA) recommends a maximum concentration of 50 ppm for a daily-8-hour exposure, which corresponds approximately to 90 mg/cu m of air. Recently both the Environmental Protection Agency (EPA) and the Food and Drug Administration (FDA) have been concerned with the health hazards associated with ethylene oxide. The concern mainly is focused on the large-scale industrial use of ethylene oxide gas for sterlization of medical supplies and equipment. While a 3 vol-pct amount of ethylene oxide in air is flammable, the first warning of its presence is eye and nose irritation, which could result in nausea or a numbing of the sense of smell. Accidental over-exposure of humans to high concentrations of the compound have resulted in nausea, dizziness, and signs of mental disturbances. In general there were no reported deaths of mice, rats, guinea pigs, cats, dogs, and rabbits at ethylene
oxide exposure levels below 150 ppm. The recent concern over the toxicity of ethylene oxide gas and the fact that the polymer used in the Bureau’s flocculation dewatering operation has an ethylene oxide base prompted this study of the properties of both the monomer and the polymer.
Polyethylene Oxide as a Flocculant for Clay
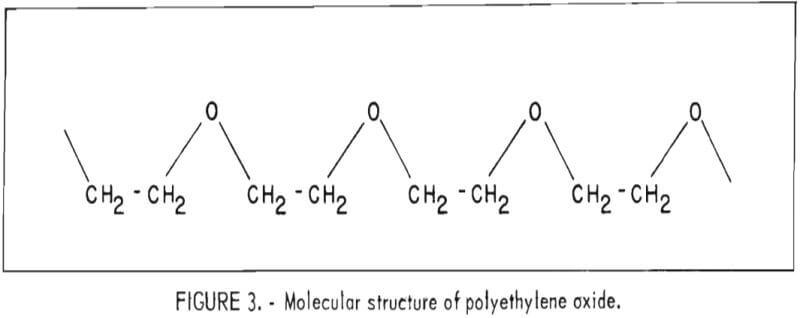
The polymerization of ethylene oxide involves the opening of the highly reactive and strained three-membered ring to form a much more stable and less reactive chain structure. This is an addition reaction that results in molecules comprised of repeating [-CH2-CH2-O-] units as shown in figure 3.
The polyethylene oxide polymer used in tests by the Bureau of Mines is Polyox Coagulant grade manufactured by Union Carbide Corporation and referred to as PEO in this report. It is a nonionic, water-soluble molecule and has a molecular weight of 5 million amu.
Polyethylene oxide can be degraded by both chemical and mechanical means. Autoxidation is the primary means of chemical degradation. Strong acid, oxidizing agents such as oxygen and hydrogen peroxide, metal ions, heat, and exposure to light, especially in the ultraviolet region, all contribute to the chemical degradation. Mechanical agitation and shear degradation also produce chain scission. This mechanical degradation is not unusual; it has been reported that polymers with oxygen bridges in the molecular backbone have higher degradation rates than polymers with C-C bonds only.
Thermal degradation studies over a temperature range of 70°-200° C on polyethylene glycols in vacuo or in solution under nitrogen resulted in chain cleavage that produced lower molecular weight molecules. There was no evidence of any weight loss or evolution of volatile products. The infrared spectra and chemical analyses of the degradation products were identical to those of the starting material. Air oxidation at 130° C of a polyethylene glycol with an initial molecular weight of 21,900 amu produced further chain scission, resulting in a molecular weight as low as 2,600 versus a lower limit of 16,200 found during vacuum degradation. This is presumably due to the
presence of peroxide units on the chain. Other studies of thermal oxidation products from a polyethylene oxide of about 10 amu molecular weight in an oxygen atmosphere between 20° and 160° C identified these degradation species: H2, CO, CO2, H2CO, H2O, HCO2CH3, and CH3CHO.
All degradation studies have shown that lower molecular weight polymers containing ether linkages are formed. In extreme environments such as pyrolsis, formaldehyde, acetaldehyde, carbon dioxide, and water are also formed. But unlike polymers of aldehydes, which decompose back to the monomer, there is no evidence that polyethylene oxide decomposes into ethylene oxide.
High-molecular-weight polyethylene oxides and their normal degradation products of lower molecular weight polymer units have been tested extensively and found to be nontoxic to mammals and marine animals. The Naval Undersea Research and Development Center published a study on the toxicity of polyethylene oxide and polyacrylamide polymers and their degradation products. They concluded the polymers were nontoxic and did not have an adverse effect on the environment. The results of other investigators were the same.
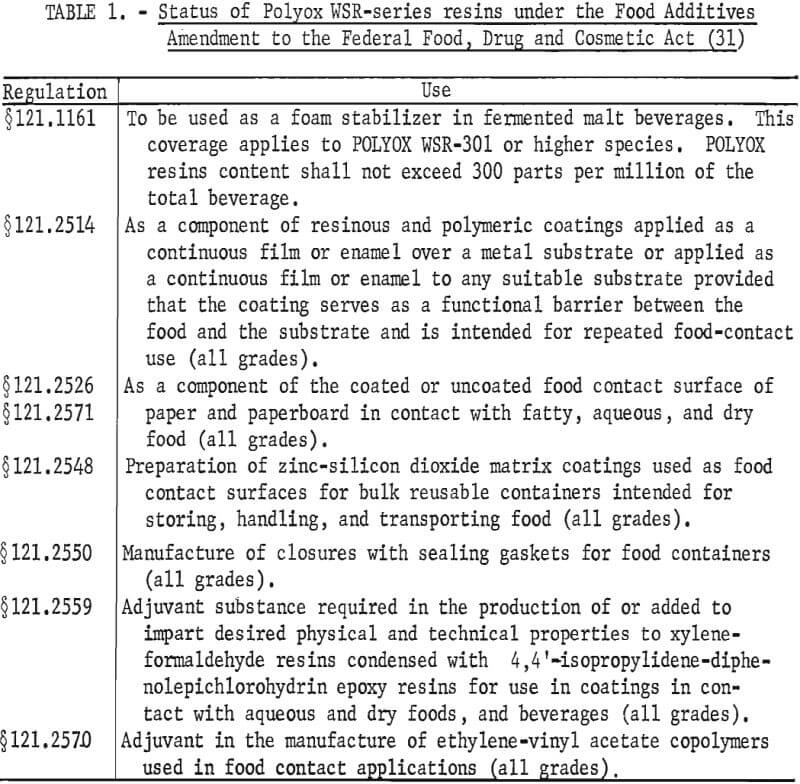
The FDA has approved the use of Polyox polymers in food, food preparation techniques, and food packaging materials as summarized in table 1.
The amount of PEO used in the Bureau’s flocculation dewatering operation is calculated to be within FDA approved dosages. A level of 1.5 lb PEO/ton of dry clay solids is normal. The dewatered clay product at a solids content of 40 pct and containing all of the PEO would have a PEO content of 300 ppm, the same amount that can be contained in beer with FDA approval.
Polyethylene oxides also are approved for use in a number of human contact products in the drug, cosmetic, and agricultural industries. Some examples are: denture adhesive, pill binders, tablet coatings, suppository bases, toothpastes, cosmetic creams, lotions, soaps, and “seed tape” that assures planting uniformity. EPA has approved unrestricted use of Polyox polymers as inert ingredients in preharvest pesticide formulations.
Description of Clay Waste Material
Three types of waste materials, a phosphatic clay waste, potash clay waste, and tailings from an aluminum leaching sequence were used in this study. The phosphatic clay waste was obtained from an operating process plant in central Florida. The potash clay waste sample contained 20 pct solids and was obtained from an operating potash plant near Carlsbad, N. M. The third waste material used in this study was tailings resulting from the leaching of kaolin clay with HCl to recover alumina. This product was produced in the Bureau of Mines alumina miniplant operation at the Boulder City Engineering Laboratory in Boulder City, Nev.
 Experimental
Experimental
There are a number of analytical methods available for determining ethylene oxide. A rapid and sensitive method involves the separation and detection in a gaseous mixture, such as air and ethylene oxide, by gas chromatography. This was the analytical method chosen for this study.
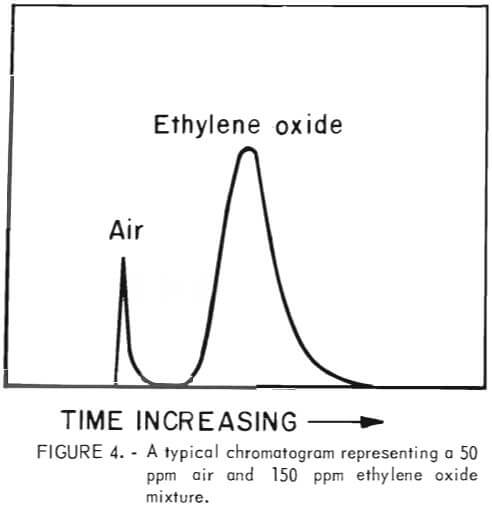
To effect a separation of air and ethylene oxide gas a 5-foot by 1/8-inch chromatographic column was packed with 20 pct squalene adsorbed on Chromosorb G packing material. The chromatograms were run at ambient temperature on a Varian Aerograph Model 1520 B gas chromatographs using helium as the carrier gas. A cylinder of high-purity ethylene oxide was obtained from the Matheson Company. This was used to prepare standard chromatograms by injecting known concentrations of ethylene oxide into the chromotograph and determining the area under the peaks. The air peak comes off the column before the ethylene oxide peak. A typical chromotogram, representing 150 ppm of ethylene oxide, is shown in figure 4. This system had a detection limit of 25 ppm.
Various combinations of PEO and waste materials were prepared and are described in table 2. Compost material was added to simulate an agricultural environment where waste material was used as landfill. Water slurries were used in the samples since the polymeric flocculant is added as an aqueous solution to the clay waste slurry in the dewatering operation. Although ethylene oxide is water soluble, it is a highly reactive species and the three-membered epoxy ring can be readily opened in water as shown in figure 2. The environmental and health hazard of concern in this study was the release of ethylene oxide gas to the atmosphere where polyethylene oxide was used as a flocculant. Small-mouth Pyrex bottles were used to contain the samples. Rubber stoppers with sleeves (septums) sealed the bottles. The needle of a gastight syringe was able to penetrate the center of the septum, which then sealed on removal of the needle. Figure 5 shows an operator taking gaseous samples for chromatographic analysis.

All samples containing PEO were prepared in duplicate. Samples were taken periodically over a 75-day period and analyzed by the gas chromatographic technique. The sampling schedule is shown in table 3. An air sample also was run for comparison each time samples from the systems were tested. After 75 days the rubber septums were worn at the place of injection by the syringe, and testing was terminated.

Results
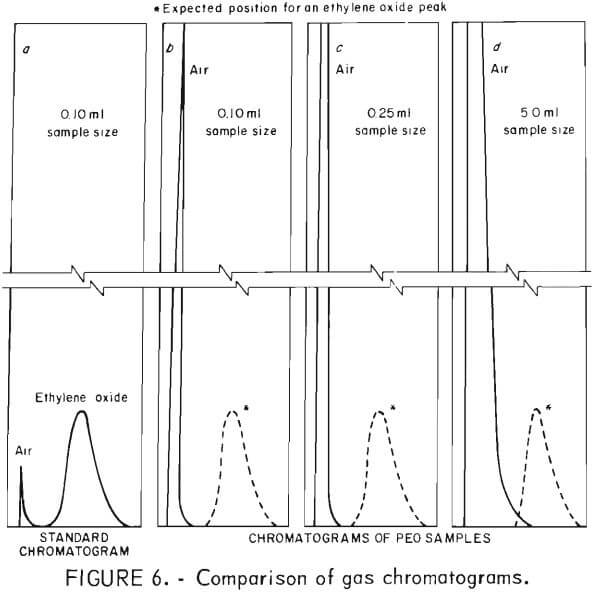
Chromatograms of all samples, taken over 75-day period, were identical with air and showed no separation of an ethylene oxide peak. The possibility of trace amounts of ethylene oxide being present and not detected also was evaluated. Standard chromatograms, prepared by injecting known amounts of ethylene oxide and air, established the distinct and identifiable positions of the air and ethylene oxide peaks. Figure 6a illustrates how well separated the two peaks were. The strip chart recorder that printed the chromatograms was equipped with an integrator which allowed simultaneous integration of the area under the peaks with the recording of the chromatograms.
Sample sizes of 0.10, 0.25, and 5.0 ml were used for chromatographic analysis. Typical chromatograms are shown in figures 6b, c, and d. With the proper attenuation of the chromatograph and the use of the 5.0-ml sample size, it would have been possible to detect trace amounts of ethylene oxide on the order of 25 ppm, well below the 50 ppm maximum concentration recommended by OSHA.
Each time sample chromatograms were run, an air sample was run for comparison. Only one peak appeared in each and every chromatogram of the 22 different samples prepared for this study. This peak was recorded in the position of the air peak, and there was no evidence of a peak at the position where ethylene oxide would have appeared. The only difference in the chromatograms of different samples sizes was the height and width of the air peak.
Conclusions
Ethylene oxide gas was not detected in systems that simulated disposal environments of clay wastes flocculated with PEO. This study substantiates degradation studies on polyethylene oxide performed in other laboratories and reported in the literature. After consideration of the literature and experimental data, it was concluded that adverse environmental effects were not likely to result from the use of polyethylene oxide for flocculating clay waste products.
