Table of Contents
- Clay Mining & Beneficiation Procedures
- Early Dewatering Experiments
- Phosphatic Clay Characteristics and Flocculation
- Early Dewatering Techniques
- Freeze-Thaw Techniques
- Crust Development
- Overburden Pumping Test
- Dewatering With Moving Screens
- Sand-Clay Sandwich Process
- Andco Process
- Sand Wick
- Swift, Inc., In-Line Sand-Clay Mixing
- Techniques Under Current Evaluation
- Estech Sand-Clay Mix Using The Enviro-Clear Thickener
- Dredge-Mix Process
- Dredge Process
- Sand Spray Process
- Developing Technology
- Gardinier Inc. Process
- Bureau of Mines Rotary Trommel
The Florida phosphate industry currently produces more than 80% of the total U.S. supply of phosphate rock. The production of this critical mineral is accompanied by the generation of large quantities of dilute phosphatic clays. The disposal of these phosphatic clays has been a problem to the Florida phosphate Industry since the introduction of large-scale earth-moving equipment such as draglines in mining and the use of flotation processes in ore beneficiation in the industry in the 1920’s, which resulted in much greater recovery, increased efficiency, and lower costs. As the needs of the world’s farmers for fertilizer increased, the Florida phosphate industry expanded to meet the demand. As the Florida phosphate industry grew, the problem of disposal of the phosphatic clays grew proportionately through the years and today represents probably one of the mining industry’s largest waste- handling problems. The phosphate industry eventually found that the most practical way to handle the clays was to store them in large impoundments where they were allowed to slowly dewater naturally. This method was chosen on pragmatic and economic grounds; it worked and at an acceptable cost.
As the industry underwent its vast expansion during the last 20 yr, the clay management problem grew in size and importance. At the same time, the industry came under increasing attack from environmental interest groups and under increasing attention from local, State, and national regulatory agencies. The conventional clay settling areas or ponds presented several problems. They were aesthetically unpleasing, particularly in their early stages of development. Storage of the plastic phosphatic clays behind dams presented the ever-present (if actually extremely remote) possibility of a dam failure. The land used for clay storage areas meant land withdrawn from other uses and the loss of considerable acreage in a region where land values are increasing rapidly. Immense amounts of water were tied up in the clays that were desired for alternative uses, agricultural, residential, industrial, and even for further phosphate mining and processing.
The phosphate industry, as well as the Bureau of Mines, private consultants, and university researchers, have undertaken extensive research and development pro¬grams to seek a solution to the clay disposal problem. The industry has been motivated both by a public spirit and by the economic imperative to reclaim expensive land and water for alternative uses. Further inducement was provided by the legal imperative to reclaim after the enactment of the Florida Severance Tax Act of 1972.
Until 1972, the industry research effort was largely the product of the work of individual phosphate companies, and the information was usually proprietary. In 1972, through the combined efforts of the phosphate industry and the Bureau, the Florida Phosphatic Clays Research Project (FPCRP) was established. The FPCRP was organized to coordinate the phosphate Industry’s activities, under the direction of L. G. Bromwell, working in concert with the Bureau. University researchers, private consultants, and other governmental agencies were also involved. In 1979, the Florida Institute of Phosphate Research (FIPR), a State agency funded through a portion of the severance tax on phosphates, was formed and became an important center of research on phosphatic clays.
Through the years, numerous techniques to dewater phosphatic clays have been developed, which varied greatly in their efficiency and feasibility. Technical practicality has proven to be the biggest obstacle to developing effective dewatering techniques. In the last decade, a growing awareness of environmental issues has caused an increased interest in the phosphatic clay problem and has led to the involvement of environmental and regulatory groups from outside the industry. All of these factors have increased the pressure upon researchers for an economic, simple, quick, and environmentally safe dewatering technique.
It would seem that every conceivable dewatering technique has been suggested and that most of them have been tried at one time or another. Those that met the test for technical feasibility were then usually tested on a larger scale. During the same time period, parallel studies of a more fundamental nature have been carried out on the clays.
This report is the result of a detailed review of phosphatic clay dewatering research by the FIPR, under Bureau memorandum of agreement 14-09-0070-954, and describes research conducted by industry, academia, and government.
Clay Mining & Beneficiation Procedures
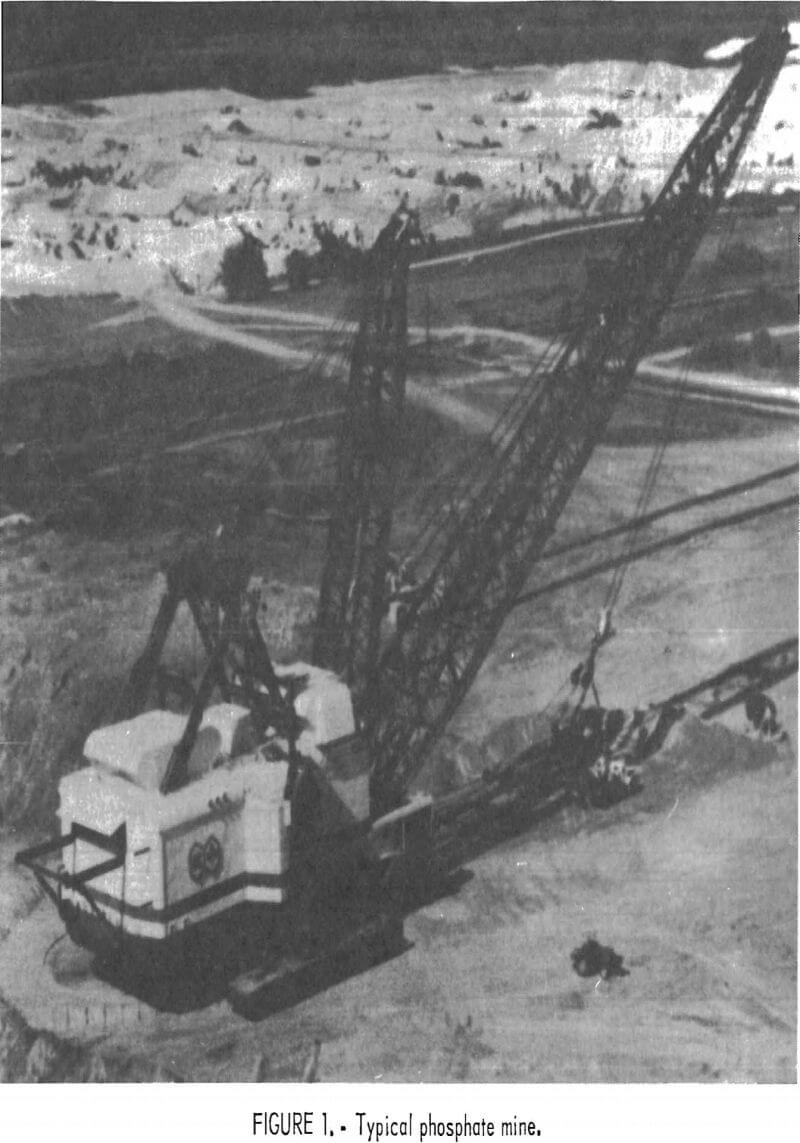
Strip mining techniques are in general use in the Florida phosphate industry, as shown in figure 1. Large draglines are used to dig a series of parallel cuts, which are each several hundred to several thousand feet in length and 200 to 300 ft wide. The overburden is cast into a previously mined parallel cut, and the phosphate-bearing zone (matrix) is exposed. The matrix is mined by the dragline and placed in a slurry pit, located above ground level and in reach of the dragline. In the slurry pit, large, high-pressure water guns (10,000 to 12,000 gal/min at 200 lb/in²) break down the matrix to produce a slurry (fig. 2), which is then pumped to the beneficiation plant.
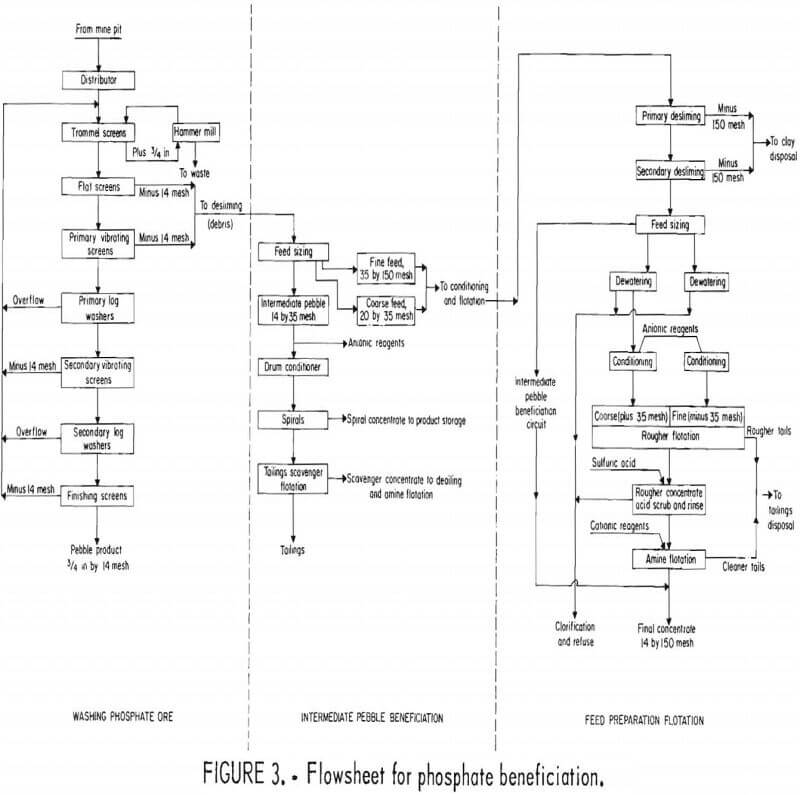
The method of phosphate beneficiation depends upon the type (size) of material that constitutes the slurrified feed. Phosphate pebble (fig. 3) is separated by a series of screens and log washers, or their equivalent, in a closed circuit. The pebble-size concentrate is utilized directly, while the finer material (minus 35 plus 150 mesh) is conditioned and flotation reagents are added. The clays and sands are separated from the final phosphate concentrate as waste tailings.
Large quantities of clay waste are produced in the beneficiation process (fig. 3). A typical company, producing 3 million ton/yr of phosphate product, mines about 400 acres of land, removing 13 million yd³ of overburden, and produces 9 million yd³ of matrix. For each ton of phosphate produced, approximately 1 ton of sand tailings and 1 ton of clays must be disposed of. These figures vary widely, however, depending upon the nature of the phosphate matrix. The clays are dispersed in water as a dilute colloidal suspension, and a typical mine can generate many millions of tons of phosphatic clay tailings annually. Each phase of the mining and beneficiation operation contributes to the generation of phosphatic clays. Collectively, phosphatic clay is composed of minus 150-mesh particles of clay, quartz, and phosphatic materials that are rejected during beneficiation. Generally, phosphatic clay slurries containing 3% to 5% solids are impounded behind dikes, which are built around mined-out pit areas. Approximately 60% of this material is stored below ground and 40% above ground level. These dikes often range in height from 20 to 60 ft above natural ground level and occupy as much as 300 to 800 acres each. Figure 4 shows an aerial view of a typical
settling pond. It is estimated that each year 2,500 acres of additional storage area are needed for phosphatic clay disposal.
Early Dewatering Experiments
Many novel technologies have been studied in an effort to solve the phosphatic clay disposal problem. Among these have been numerous electrical methods.

Studies of the electrophoretic characteristics of phosphatic clays have been undertaken by several private investigators as well as by the Bureau. Electro-osmosis and electrophoresis methods have been investigated for their possible use on phosphate clays. The techniques have been applied for some time to fine particle slurries, particularly as an aid to filtration or to dewater fine slurries underground. Attempts to use electro-osmotic techniques to dewater phosphate clays date back over 20 yr. While the technology “would appear to have promise,” it is “not considered commercially feasible” except under special circumstances because of the slow dewatering rate of the clay wastes. Significantly increasing the dewatering rate is possible but involves prohibitive energy costs. Bureau investigators have determined the electro-osmotic characteristics of fine phosphate clay wastes and were able to dewater clay wastes up to 35% solids on a small (4-ton) scale. The power required to increase the solids content of the phosphatic clays from 17% to 25% was 25 kW-h/ton of water removed. The (DC) voltages used in the tests ranged from 8.8 to 245.5. As the clays thickened, higher voltages were required and power requirements also increased. There, was also difficulty in removing the supernatant liquid from the clay wastes as the clay thickened and the migration of the water became more difficult.
Electrostatic beneficiation of phosphate ore was investigated by International Minerals and Chemical Corp. (IMC) in the mid-1950’s with a view toward eliminating the need for the flotation process. Investigators at Princeton University Plastics Laboratory investigated the potential use of nonuniform electrical fields for the continuous separation of suspensions in a dielectric fluid, water. The Tennessee Valley Authority (TVA) built and tested a rotating anode machine, and the Bureau built and tested a continuous electrical dewatering system using a moving metal belt as the anode. These reduced the moisture content of the clay suspensions but failed to yield clear effluent water.
Magnetic separation techniques were applied to phosphate ores by a Massachusetts Institute of Technology (MIT) group in 1972 and 1973, with a view toward avoiding the creation of phosphatic clay wastes. The process did not prove feasible. Scientists at TVA, Virginia Polytechnic Institute, and IMC investigated the possible use of ultrasonic energy to precipitate phosphatic clays as early as the 1950’s. However, a commercially feasible system was not developed at the time. In recent years, there has been a renewal of interest in the possible use of ultrasonic energy to precipitate clay slurries.

An attempt was made in the early 1950’s to use cull citrus fruit, which represented a large volume of waste, to mix and react with phosphatic clays and to solve a mutual disposal problem. The volume of clays produced by phosphate mines is so large that the process proved impractical without even considering other difficulties such as transportation.
The use of continuous centrifugation processes to dewater phosphatic clays was investigated by TVA and others. The use of centrifuges was determined to be impractical , however, because of high capital investment and energy requirements.
Beneficiation of phosphate ore by using dry methods was investigated by a private company as a possible means of eliminating the production of dilute phosphatic clays as a byproduct. The process developed was not adopted because it proved impractical.
Phosphatic Clay Characteristics and Flocculation
Through the years, numerous studies have been made of the mineralogical, physical, and chemical characteristics of phosphatic clays. These studies have largely been undertaken by industry itself, by the Bureau, or by private or university consultants sponsored by the industry, the Bureau, FPCRP, or FIPR.
Phosphatic clay samples taken from settling ponds were characterized by Bureau- sponsored researchers at Florida State University using an electron microscope. The clays were examined to determine the shape and texture of the clay particles and how these affected their flocculation and settling in disposal ponds. This was basic research aimed at understanding the fundamentals of clay particle behavior and is typical of numerous characterization studies of phosphatic clays undertaken by various investigators. Other characterization studies also described the variations among the minerals making up the phosphatic clays throughout the phosphate mining district.
C. C. Ladd of MIT undertook basic consolidometer and permeability tests on representative samples of Florida phosphatic clays. Tests were also made in a test pit using various combinations of drains, consolidation rates, and pore pressure measurements, and to determine the effect of hydraulic gradients.
Screening of possible flocculants for use in dewatering phosphatic clays has been undertaken at various times by private companies, consultants, and the Bureau. Flocculation is a technique in which discrete, colloidal-sized particles are agglomerated by an appropriate reagent and, as a result, settle out of suspension. Hundreds of commercial flocculating reagents have been tested singly or in combination with others, in an effort to select a flocculant that will result in the formation of stable flocs that will not reslurry readily and that will cause rapid settling and dewatering of phosphatic clays. Frequently, successful flocculating reagents evaluated in the laboratory on a specific clay proved unpredictable. In field tests owing to the variables encountered in the field test conditions. Among these variables are clay mineralogy, age of the clay slurries, the method of flocculant introduction, the dilution of the clay slurries, the pH of the slurry, the mixing shear, and the conditioning and contact time. A systematic evaluation of the many hundred available commercial flocculants was undertaken by the Surface Chemists of Florida (SCF), in research sponsored by the FPCRP. SCF found that high-molecular-weight organic polyacrylamide polymers were the most efficient but that galactomannans (guar) were about as good. It was found that while high-molecular-weight polymers were better flocculants, a point of diminishing return was soon reached with increasing molecular weight. Anionic flocculants proved superior to neutral or cationic ones, and combinations of polymers were often more successful than single ones. In fact, combinations of poor flocculants together were extremely effective in some cases.
Several processes using chelating agents to hasten the settling of phosphatic clays have been investigated, and some have been patented. None appear to have been adopted on a practical scale.
Basic studies of the mechanism of flocculation of phosphatic clays were undertaken by researchers at Auburn University for the Bureau. The effect of the compressive stress exerted by a column of clay wastes was first studied, and then small-scale models of thickening systems (fluid bed and the Lamella thickener) were built and tested. The conclusion tentatively drawn from the tests was that several thickeners used in series would result in effective and efficient dewatering of phosphatic clays.
A group of researchers at MIT, led by C. C. Ladd, in work sponsored by the FPCRP, found that phosphatic clays solidified in four stages or phases. These all occurred simultaneously but differed in degree of importance during the time progression of settlement. The first was sedimentation, in which the clays settled out of the supernatant water. Then came consolidation, in which the clays solidified under their own weight, squeezing out entrapped and interstitial water. Consolidation generally followed Terzaghi’s theoretical model. Next came the stage of consolidation under hydraulic gradients. If the pore pressures at the bottom of a column (or depth) of consolidating phosphatic clays were reduced below hydrostatic levels, seepage would occur as more supernatant liquid migrated down the column to equalize the pressure. This led to a formal recognition of a fact already known in practice: that bottom drainage from a phosphatic clay holding area above natural ground greatly enhanced the dewatering of the clay. The last stage was that of surface stabilization. The presence of surface water tended to keep the surface weak. Removal of this water by promoting surface drainage led to greatly enhanced drying and to the creation of a surface crust sufficient to bear weight. The most important idea brought out by the MIT-based researchers was probably the critical role of a column of weight pushing down and enhancing the consolidation of the phosphatic clays.
Colloidal gas aphrons (CGA) are dispersions of micrometer-sized bubbles that can concentrate the phosphatic clays in the dilute waste stream by a combination of flocculation and flotation. This process was evaluated by researchers at Virginia Polytechnic Institute under a grant from FIPR. The technique of using CGA to minimize the water content of the clays and to separate phosphate values from the clays is being investigated, and various flocculants are being evaluated for cost and effectiveness.
FIPR also funded Zellars-Williams, Co., to study the effect that the addition of hydrated lime had upon phosphatic clay dewatering. It has been known that the addition of lime enhances water recovery and improves the material strength of the remaining solidified material. This study aimed at quantifying the effect of lime addition to dilute phosphatic clay and trying to determine the final amount of stabilization possible in dewatered clays. The effect of residual organic flocculants and the addition of sand tailings or phosphogypsum to the lime treated clays were additional parameters that were studied.
IMC, with FIPR support, is testing a method of disposing of phosphatic clays in old mine cuts. The partially dewatered clays are pumped into the mine cuts. The overburden windrows give good access to the disposal area and make less difficult the task of placing a sand or soil cap on the clays after they have dewatered further. The mine cuts also present a greater surface area for dewatering drainage than conventional disposal ponds. A full-size test pit was filled and evaluated.
Basic chemical, mineralogical, and mechanical characteristics data were collected by Bromwell Engineering for an FIPR project. Phosphatic clay samples were taken from disposal areas, current mining operations, and locations designated for future mining. The tests included chemical analyses (P, U, Ra, Al, Fe, Mg, F, Ca, and cation exchange capacity), mineralogical analyses (X-ray diffraction and scanning electron microscopy) , and physical analyses (grain-size distribution, plasticity indexes, viscosity, settling characteristics, and slurry consolidation behavior). These data, when analyzed and published, will provide a foundation of clay characteristics for future research.
Research was undertaken by the Bureau and by private and university investigators under Bureau sponsorship to see if micro-organisms could be used to promote aggregation of clay particles from dilute phosphatic clay wastes. Some fungi were found to be effective but only when their growth had been greatly enhanced by adding nutrients to the clay slurry. This was judged to be an impractical process at the present time.
Numerous investigations have been made by the Bureau, State laboratories, university scientists, and private companies of the use of ion-exchange techniques in dewatering phosphatic clays. In numerous similar industrial processes, ion-exchange technology has proven successful. However, to date no ion-exchange process has proven practical.
Early Dewatering Techniques
Several dewatering techniques exhibited enough promise at the laboratory scale that large-scale tests were carried out in the field.
Freeze-Thaw Techniques
Freeze-thaw techniques have been used successfully on a small scale to dewater fine clay wastes and sludges. When the wastes are frozen, the water separates from the clay particles and freezes as ice. Upon thawing, the clay particles remain dehydrated and settle as a relatively high-density concentrate from which most of the water can be decanted. Bureau investigators found that phosphate clays averaging 13.7% solids can be quickly frozen and thawed to produce a settled clay fraction comprised of 42% solids. The energy costs were calculated at 183 Btu/lb of clay wastes. At that time, industry found these energy requirements to be “prohibitively high,” and the technology to treat the large areas required has not been developed.
NTP Corp., under a grant from FIPR, is currently testing a thermal process for the rapid dewatering of phosphatic clays. The system Is a freeze-melt one using n-butane in a vapor compression refrigeration cycle to freeze-separate the colloidal clay-water suspension. Prethickened phosphatic clays (18% solids) are pumped through a precooling heat exchanger and into a freeze tank where liquid n-butane is bubbled through the clay slurry. The expanding n-butane cools the slurry, and ice forms and is removed. The n-butane is recovered and recycled. Two methods for removing the thickened phosphatic clays are under study: In one, the clays move with the ice slush and are separated in the melting water cycle; in the other, the thickened clay solids are removed from the bottom of the freeze chamber.
Crust Development
Several approaches have been pursued with the aim of producing a crust upon waste clay settling ponds capable of bearing a substantial weight. If sufficiently hardened, in a reasonable time, the crust can then support equipment capable of spreading other, more permanent surface materials such as sand tailings and overburden. One approach that has been successful is to allow a vegetative cover to appear. This is usually comprised primarily of cattails. The vegetative surface offers much greater bearing capacity than the clay surface although it is relatively slow to become established. Encouraged by these results, IMC systematically sought out and tested various grains and grasses for their growth potential as well as for their shear strength properties. IMC found that Japanese millet thrived in the phosphorous-rich clays, was able to tolerate the watery environment, and produced a bearing surface of great strength in a short time. In 10 weeks, the Japanese millet grew to a height of 2 ft and had an average root depth of 1-½ ft. Furthermore, it naturally reseeded itself and displaced the other test species in the pit.
Efforts to aid the desiccation of the clay settling area surface itself were also undertaken. Mobil Chemical Co. attempted to apply a method that had been successful elsewhere in dewatering fine particle sludge, the Hardaman crust disruption system. The fine suspensions (clay wastes in this case) are pumped into wide, shallow holding areas where they are allowed to settle. The clear water is drawn off, and the remaining solids dry in the air and sun. When a crust has formed, tractors are used to break up the surface and expose new material to drying. However, this method was found not to work with Florida phosphate clays because of their slow settling rate, the high ambient humidity, the heavy rainfall, and the vast areas of land that would be required.
Currently, efforts are underway to develop specialized equipment for use on a partially solidified clay settling pond surface. One problem in reclamation of mined-out areas has been the long time periods needed to develop a surface crust sturdy enough to support equipment that can cap the wastes and thereby hasten the return of the mined-out area to other usage. Special high-flotation vehicles are being developed, and at least one company is experimenting successfully with the use of such equipment to spread sand tailings over the semisolid surface of a clay waste settling pond.
Overburden Pumping Test
In 1972, Mobil Chemical Co. undertook tests using slurrified overburden to cap clay wastes. Based on preliminary laboratory data, an 80-acre test site was established in a mined-out pit. Overburden from an active mining site was slurrified and pumped to the test site where it was mixed with the phosphatic clays from the phosphate processing plant. The tests were not successful. There were several reasons for the failure. The overburden included quantities of clay, which, when slurrified, produced even more fine suspended clay particles and made the problem of disposal worse. The overburden and phosphatic clays tended to separate and the clay to migrate. The overburden entrapped very little clay. The slurrification process also put into water suspension organic materials, which formed very stable suspensions. The resulting water turbidity required a separate clarification process.
Dewatering With Moving Screens
Bureau researchers investigated the use of moving screens as a potential method of dewatering phosphatic clay wastes. The method was based on the idea that if the gel-like structure of the colloidal clay wastes suspension were gently broken up mechanically, it would free a large percentage of the interstitial water at an acceptable cost.
Laboratory tests were performed on 18 industry-supplied phosphatic clay waste samples. These samples, from different plants, varied in composition and ranged from 2.6% to 16.7% solids content. Small screens (4, 8, or 16 mesh) were slowly moved through the 250-mL columns of clay wastes, and the supernatant water was periodically removed. Similar tests were carried out on 8-gal samples using the same screen sizes (4, 8, or 16 mesh) but arranged so that there were three screens (of the same size) mounted one above the other on a shaft.
The moving screens effectuated significant dewatering in a relatively short time period. Typical samples, with 4.7% and 11.9% solids content, dewatered to 16.8% and 25.2% solids content, respectively, in 3½ days. An attapulgite sample (2.7% solids content) dewatered to 13% solids in the same time period.
One interesting effect, of possible industrial interest, was that dewatering extended significantly beyond the volume through which the screens moved. Screen size was found to have no significant effect; the 4-, 8- and 16-mesh screens produced roughly equivalent dewatering. The speed at which the screens were moved proved, however, to be a critical variable. To achieve significant dewatering, the screens had to move with extreme slowness. Significantly, it was seen that clay wastes that had naturally poor settling characteristics, such as attapulgite, produced higher percent solids as a final product by moving screen compaction than those that settled more rapidly under normal conditions. It was also found that the most dilute samples (lowest percent solids content) lent themselves to the greatest degree of dewatering by the moving screens.
The process appears to be too slow, however, to cope with a large flow of clay wastes but might be suitable for special usage with particularly difficult clays.
Sand-Clay Sandwich Process
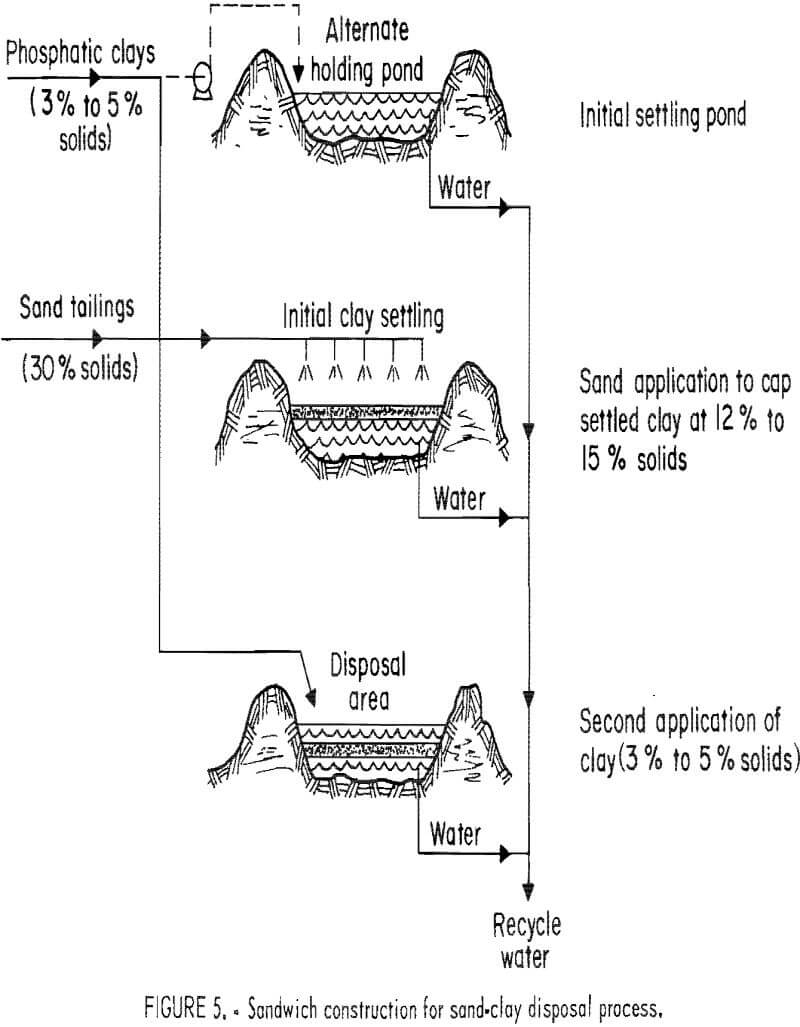
The difficulties of mixing sand and clay led to experiments in which alternate layers of sand and clay were put down, with the sand tailings layers providing drainage paths and putting weight on the phosphatic clays below, which aid dewatering.
A test site was created at USS Agri-Chemicals Rockland Mine. The test pit was 40 by 5 by 7 ft deep. It was found that it took about 30 days for the 3.5% solids content of phosphatic clays to reach a consistency that would support a gently emplaced sand tailings layer (17% to 23%). Phosphatic clays (3.5% solids) were added to the 7-ft test pit in layers 4 to 6 ft thick. Within 30 days these had naturally dewatered to such an extent that they remained only 7 to 19 in thick. Sand tailings were then gently added in thin layers. The experimenters were careful to connect the sand layers to the sand dam at the outlet end of the pond so as to provide an adequate drainage path (fig. 5). An equivalent total depth of 42 ft of clays averaging 3.5% solids content were added, through a 308-day period, to the 7-ft pit. The final thickness of the clay bodies was 42 in, and the clay solids averaged 33.8%. The design of the experiment was aimed at creating a 400- to 600-lb/ft² pressure on the clay wastes. This should give a final clay solids content exceeding 35%, which occupies a small enough volume that all the beneficiation waste products can be stored below grade level. The weight is provided by the alternate sand and clay layers pressing on those below. Later tests were carried out using a floccu-lated clay-sand mixture (1:1 by weight) in order to shorten the period needed for the clays to dewater sufficiently to support the sand layer. This resulted in a clay-sand mixture in which the clay fractions changed from 3.5% to 31.5% solids in 133 days.
The results of the tests were favorable, but there were problems. The turn around time for placing each layer of clay is long, about 1 month. As each layer is relatively thin, this means that large areas must be available to store the phosphatic clays. The mechanics of the operation present problems. The sand must be placed in thin (6-in) layers uniformly over a wide area. Some sort of special system or machinery would have to be devised to cover more than a small-area. The test pit was small enough that this was not a major factor. Slow drainage through the sand layers was also a problem, particularly because of the large distances (300 to 500 ft) that would be involved in an actual operation.
Andco Process
ANDCO, Inc., developed a process for dewatering clay tailings, which was tested in cooperation with USS Agri-Chemicals and IMC. The ANDCO process was based upon the use of a proprietary poly-electrolyte flocculant identified only as A-802, which had been developed after lengthy testing. A 2-week test of the
ANDCO process was carried out at the USS Agri-Chemicals Rockland Plant site at Ft, Meade, FL, in September 1973. Besides supplying the site, phosphatic clay, and sand tailings, USS Agri-Chemicals provided some equipment and labor. The clays were pumped into a 30,000-gal thickener, the flocculant A-802 was added, and the flocs were allowed to settle. At this point, the phosphatic clay underflow ranged from 5.5% to 13.1% solids. The underflow was pumped to a screw conveyor. Tailings sands and additional flocculant, A-802 also, were added at the input hopper of the screw conveyor. The sand-clay mixture was fed into a modified drum conditioner. More flocculant was added to the drum conditioner as needed to “toughen” the agglomerated mixture as it passed through. The sand-clay mixture was then pumped to a commercial belt filter unit. The filter unit used a combination of pressure and filtration to produce a resulting solids content of 55% to 70%. Flocculant-laden underflow water from the filter unit was recirculated to the primary thickener tank. The ANDCO process aroused little interest in the phosphate industry at the time because of what were then believed to be high costs. The system is also highly dependent upon maintaining a proper ratio of sand to clay wastes, which varies, however, with the nature of the ore being rained. This requires close control and above-average operator skills. The process did provide rapid consolidation of the clay solids and rapid recycling of the process water.
Sand Wick
In an effort to expedite the dewatering of large masses of clay wastes, several types of drainage systems were investigated. One of the most promising involved the use of sand wicks or sand column drains. Much of the initial theoretical work was done by researchers at the University of Florida, supported by the National Science Foundation and working with the Florida Phosphate Council and various phosphate companies.
In the early efforts, phosphatic clay samples were subjected to particle size analysis and the permeability characteristics were studied. A permeability cell was built, and attempts were made to develop theoretical equations that could describe seepage through a sand column. Small test cells using sand columns or wicks were built and then larger units: a 55-gal test cell and a test cell 4 by 4 by 8 ft high. Field tests, when run on a small scale, quickly revealed problems. The main one was that the sand columns did not retain their shape and cohesion. They tended to collapse or to mix with the phosphatic clays as the very fluid clay migrated. To retain the sand in a column, it proved necessary to confine it within a rigid but porous structure. Perforated PVC pipe filled with sand was tested, but burlap sacking within a wire screen framework, supported by a small pipe and filled with sand, gave the best results. This method provided enough support so that the sand columns retained their integrity, while it also provided the most intimate liquid contact possible with the clay wastes.
Large-scale testing was undertaken at a site near the IMC Noralyn washer near Bartow, FL. Two pits, roughly equal in size, were dug. One was the test pit; the other was used as a control. The test pit was 50 by 50 ft at the base, and 95 by 95 ft at the top, and 12 ft deep. The pit actually extended only about 9 ft into the ground because of the high water table; sand tailings were used to create a small wall to achieve the desired total height. On the bottom, a 1-ft layer of coarse material (¾-in gravel) was spread to provide a drainage path. In both pits a sump was fashioned from a 3-ft section of steel pipe, 20 in. in diam with a 16- by 16-in screened opening near the lower end to serve as an inlet port. Gravel was piled around the sump and then covered with sand to prevent the clays from entering the sump. A plastic pipe was used as a conduit for the suction hose connecting the sump with a centrifugal pump located on the test pit retaining wall.
In the test pit, 16 sand column “seepage aids” were placed 11 ft apart in a square array. These were made of burlap, supported by a 1- by 1-in wire mesh cylindrical casing and were 8 in, in diam and 12 ft high. The columns were filled with sand tailings, and each had an additional support, a 1-in aluminum pipe. Each was also secured by three guy lines.
When the sand column seepage aids were completed, phosphatic clays were pumped into the test pit and into the control pit. Some 476.000 gal of 3% solids clays were pumped into the test pit and 414.000 gal of 4% solids clays into the control pit.
After 190 days, the volume remaining in the test pit was 67,300 gal of 24% average solids (1 ft above the base). There were 115,300 gal of 20% average solids in the control pit, and there was a foot of free water on the surface of the control, pit, but none on the test pit surface. After 280 days, the material in the test pit, 1 ft above the base, had reached an average of 49% solids compared with only 28% in the control pit.
Using reinforced sand columns as seepage aids in dewatering clay wastes appeared to speed up the dewatering considerably. There remained some unanswered questions. The inability of the sand column to support itself meant that relatively expensive reinforcing systems would have to be used to stabilize the sand columns. The method was very difficult to apply to existing clay settling ponds. Attempts were made to establish sand column drains by sinking perforated PVC pipes into the partially solidified clays and then filling them with sand. When the pipes were removed, however, the sand collapsed or mingled with the shifting and migrating clay. It also proved difficult to slurry out and remove the clay material within the pipe so sand could be placed there. It proved impossible to remove sand tailings mixed with clay when they were encountered. The sand column or sand wick technique also suffered from the low permeability of the clay, a problem common to all water removal techniques. The phosphatic clays nearest the seepage aids tended to dry out, cake, and inhibit the flow of supernatant water from farther away.
On the positive side, the sand column or sand wick method did produce more rapid dewatering and resulted in a higher percentage solids content for the final product. It allowed surface water that stood on the pit surface to be drained, and it enhanced drying and cracking of the clay waste surface, which in turn led to further dewatering.
Swift, Inc., In-Line Sand-Clay Mixing
Swift, Inc., working with the SCF, undertook the development of in-line mixing of flocculated phosphatic clays and tailings sands beginning in September 1975, as part of the FPCRP. Work was done to determine the proper flocculant and the optimum sand-clay mixture. It was soon found that static, in-line mixers did not give the desired results. The sand and clay fractions tended to separate, and there were problems with inadequate flocculation of the clay fractions. The effects of shear during the mixing also promoted segregation. These tests led Swift (later Estech General Chemicals Corp.) to adopt the use of thickeners to prethicken the phosphatic clays, a process described below.
There are numerous variations of the general method of using sand tailings to mix with dewatered phosphatic clay tailings in order to both dispose of mine wastes and reclaim mined-out areas. These different systems are presently being evaluated by several phosphate companies. They differ in some or many particulars: for example, the use of special flocculants, the use of special thickeners or more than one thickener, and the use of special techniques as part of the process, such as vibratory or rotating screens.
Techniques Under Current Evaluation
Estech Sand-Clay Mix Using The Enviro-Clear Thickener
Several companies have been and some still are experimenting with the use of the Enviro-Clear clarifier-thickener to dewater clay wastes. The capacity of the Enviro-Clear system is greater than that of conventional thickeners and is more likely to be appropriate for the scale of phosphate operations.
The Enviro-Clear system is a continuous solid-liquid separation system. Chemically pretreated feed (phosphate clays treated with flocculants) is introduced into the Enviro-Clear thickener at a controlled velocity at the center of the unit, as shown in figure 6. The feed is introduced horizontally into an “active” sludge bed near the bottom of the thickener. This eliminates the free-settling zone, and the flocculated particles agglomerate and settle to the sludge zone. The process is aided by the compressive force of the thickened, sludge bed. Continuous rakes aid in compacting the solids and moving them to the discharge boot. Clear water overflows the top of the unit. There is a sharp interface between the sludge bed and the clarified effluent zone, which is considered characteristic of the Enviro-Clear device. This attribute is used to control the system through using either a vertical sight glass or an ultrasonic detector.
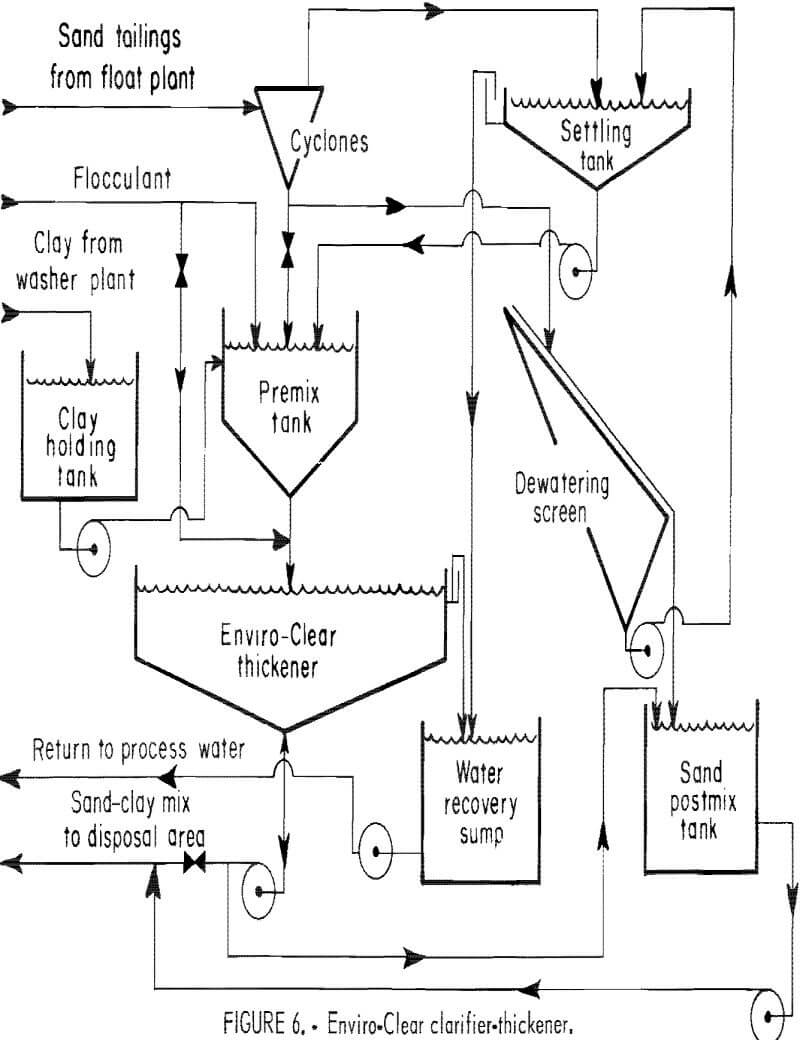
Gardinier, Inc. , rented a 3-ft-diam Enviro-Clear unit for pilot plant studies in 1977, Dilute phosphate clays were fed from a surge tank by a constant head feed so that the flow rate was constant. Flocculant was introduced into the feed stream between the feed tank and the Enviro-Clear thickener. Gardinier engineers were able to achieve a phosphatic clay output of 15.8% solids for a 0.8-lb consumption of flocculant per ton of (dry) phosphatic clays. When combined with tailings at a 1:1 ratio, this produced a mixture with 27.7% total solids. The water recovered, in-plant, from the feed amounted to 87.1%. These initial results were encouraging, and several other companies undertook parallel investigations using larger thickeners. An average-size phosphate plant processing 60,000 gal/min of clay wastes at 3% clay solids would require three 80- ft-diam Enviro-Clear thickeners. These would produce clay slurries thickened to 12% to 19% solids depending upon the composition and amount of the clay and the amount of flocculant used. Problems could be caused by the residual flocculant in the water removed from the clay slurries and recycled for phosphate ore processing. The remaining flocculant, even in tiny quantities, sometimes tended to suppress phosphate flotation. All of these expensive investments failed to increase dewatering beyond what could be ultimately achieved in conventional settling ponds over a longer period of time (15 to 30 months). There have also been difficulties reported in pumping the flocculated mixture.
The Enviro-Clear process has the advantage of rapidly dewatering dilute phosphatic clays, and it allows clay and sand wastes to be stored together in the same area. It produces a relatively stable and fertile landfill for revegetation. It has the disadvantage of being strongly dependent upon a certain range of sand- to-clay waste mixture. This is an uncontrollable factor, which depends upon the nature of the matrix being mined at a particular time. There is also the problem of residual flocculants in the effluent water, which is recirculated for mine and plant processing. Remnant flocculating agents may render the water unusable.
Recently, Estech built a plant based on the Enviro-Clear thickener, which is capable of handling the entire waste clay output of their Watson Mine. Estech chose the Enviro-Clear system because it fit well with the Estech mining system, used equipment familiar to the operators, gave a rapid return to operations of process water, and was cost competitive with conventional disposal methods. The Estech plant went into operation in October 1981. It is based upon an 85-ft-diam Enviro-Clear rapid clay thickener capable of handling 348 ton/h of solids (clay plus sand tailings) with a feed flow of 12,000 to 15,000 gal/h. The clay slurries average 3% to 8% solids. Dewatered sand tailings and the clay are mixed in approximately equal quantities with an anionic polymer in a large mixing tank. The mixture is then fed into the Enviro-Clear rapid clay thickener. Another mixing tank is provided in the process stream so that more dewatered sand tailings can be added to the thickened clay underflow as an alternative. Pumps move the thickened sand-clay underflow out to final disposal areas. Clear water overflows the thickener and is returned to plant operations.
To date, Estech is pleased with the operation of its Enviro-Clear thickener and plant. It has been able to get sufficient flocculation of the suspended clay particles to achieve a solids content of over 12% for a flocculant usage of less than 1 lb/ton (clay solids basis). Mixed with the sand tailings, this creates a homogeneous mixture that remains solid and does not allow segregation of the sand and clay fractions. Additional consolidation and dewatering in the final storage areas has exceeded initial expectations. The rapid return of process water to plant operations has resulted in “significant” savings in power costs for pumping. The resulting sand-clay mixture is also closer to normal surface soil than that produced by any other waste clay settling system and will result in a more rapid return of disturbed land to premining usage, according to Estech.
There are problems, however. The constitution of the phosphate matrix varies widely from mine to mine and even within mines. In some cases there may be insufficient sand tailings available to mix with the prethickened phosphatic clay tailings. In other cases there are large quantities of clay fractions present of the type that cannot be quickly or economically dewatered. In any case, the resulting landfill has low shear strength and is only marginally useful, after reclamation, for any purpose other than agricultural. The presence of residual flocculants in the reclaimed water may inhibit Its use when recycled for plant operations.
Dredge-Mix Process
IMC undertook the original testing of the dredge-mix method around 1975. In 1978, IMC and Agrico Mining Co., later joined by Mobil Chemical Co., undertook jointly sponsored research on waste clay consolidation methods and land reclamation. The dredge-mix process was designed to mix tailings sand with already naturally thickened clay wastes, which would be constantly pumped from the bot-tom of the initial settling pits (fig. 7). The testing was to determine if this
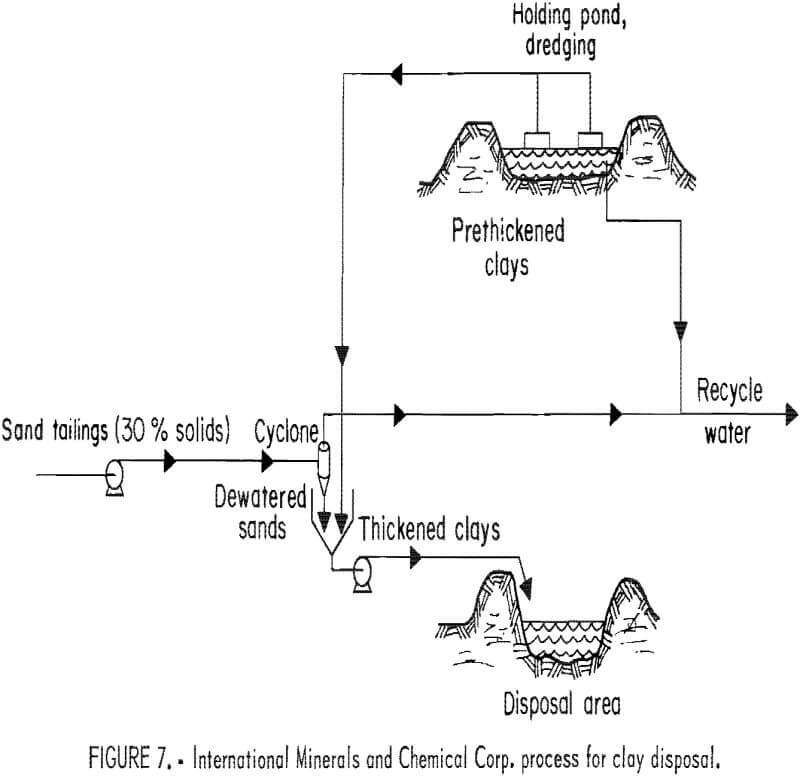
sand-clay mixture would produce land sufficiently consolidated for multipurpose uses.
Based upon research carried out at the Colorado School of Mines, a Marcona-type pump (Marconaflo) was used, which was equipped with a high-pressure rotating water jet near the intake of the submerged pump. The water jet proved superfluous, having little effect on the pumping of the clay wastes. Also, the Marconaflo pumping system was costly, and it was soon replaced by conventional submerged pumps. These proved capable of pumping the partially dewatered clay wastes as long as the solids content was under 21%. It also proved necessary to cover the intake of the submerged pump with about 8 ft of clay wastes exceeding 15% solids to prevent the preferential development of channels through lower density material.
Mixing sand tailings with the thickened clay wastes was soon abandoned by the IMC-Agrico-Mobil group when it was discovered that the addition of the sand did little to speed up the process of dewatering. Laboratory tests carried out by Mobil confirmed this.
At a later time, interest in the dredge-mix process was renewed, and CF Mining Corp. began a full-scale sand-clay mix reclamation project at its mine. The project was overseen, initially, by Ardaman and Associates, under a research grant from FIPR. The main objectives of the tests were to obtain data on the settling and consolidation of the sand-clay mix and to evaluate the nature and quality of the reclaimed land.
W. R. Grace and Co., at its Four Corners Mine, has committed itself to building a pilot dredge-mix settling area. It is planned to dredge prethickened clay from the initial settling area, mix it with sand tailings, and place it in mined-out cuts. This is approximately the same process originally attempted by the IMC-Agrico-Mobil research group.
The greatest advantage of the dredge- mix system is the ability to use it with a thickener to produce clay wastes of sufficient density to allow their rapid settling when mixed with sand tailings. The greatest difficulty is dependence of the process upon certain specific sand- to-clay ratios, when the mined matrix is actually highly variable in content.
A comprehensive comparative evaluation of sand-clay mix phosphatic clay disposal compared with disposal in conventional settling ponds has been undertaken by Ardaman and Associates, under a research grant from FIPR. The aim was to determine quantitatively and qualitatively the advantages and disadvantages of each disposal method and to determine the engineering properties of a range of sand- clay mixes and of various clays. From this, it is hoped a mechanism can be developed for prediction of sand-clay mix properties based upon the mineralogical and settling characteristics of various clays. The study was divided into three parts. In the first, the index properties of the phosphatic clays were determined. On 12 phosphatic clay and 3 sand tailings samples, selected to be representative of the whole phosphate area, soil mechanics index tests were run. These included plasticity characteristics (Atterberg limits), particle size distributions, specific gravity, activity, and viscous properties. The pH and specific conductance of the pore fluid were also measured. The samples were found to be within the range and distribution of previously published values. Mineralogical tests were also run, which included X-ray diffraction, scanning electron microscopy, and chemical analysis. From these data, quantitative estimates were made of the mineral species. It was found that clays such as kaolinite, montraorillonite, and attapulgite comprised from 40% to 65% of the phosphatic clays. Sedimentation tests were done on the 12 clay and 3 sand tailings samples. Some 22 laboratory settling tests were run. From these, the final settled solids content, settling rate, and void ratios versus effective stress relations at low stress were determined. The data were found to be consistent with the Michaels and Bolgar theory of settling behavior of clays. Few correlations were found, however, between index properties and sedimentation behavior.
Dredge Process
The IMC-Agrico-Mobil research effort redirected its attention from the dredge-mix process to the placement, by pumping, of the thickened clay wastes (15% to 18% solids content) in mined-out pits, and covering them with an evenly distributed sand cap. This has been labelled the dredge process by IMC. Lawver of IMC had calculated that it would generally require an effective stress of 600 to 800 lb/ft² on the clay wastes to dewater them sufficiently, over time, to return them to near original volume. This would allow the clay to be placed below ground level but would still require an overburden cap above the original surface contour. Placing a uniform sand cap on the clay wastes proved difficult. There were numerous failures before what appears to be a workable system was developed. Between 1978 and 1982, IMC, Agrico, and Mobil undertook sand spray tests to place a cap, under the direction of Lawver. The first attempt to use a sand spray nozzle developed by Brewster Phosphates failed. The nozzle was buoyed with floats to maintain its position near the surface. Sand tailings at approximately 30% solids were sprayed on the test pond surface at a rate of 1,000 ton/h. The spray nozzle was unable to deposit the sand uniformly, to form a cap; the sand layer, ranging from 0 to 2 ft in thickness, was heaviest near the spray. This unevenness caused the heavier sections to sink and created heaving and mud waves. Mud waving occurs when the clay surface, unable to bear the weight of the load impressed on it, either flows plastically from under the load or collapses and allows the sand load to sink, usually by sections, where the impressed load is heaviest. The term is used to describe movement of the impounded clay-sand mixture as induced shear stresses set up a heaving wave front during redistribution of the clay-sand solids.
Another attempt to deliver sand from the sides of the pond also failed. The sand was deposited only near the outlets, with consequent unevenness. A central mud wave was also created. In an attempt to solve the problem of the even distribution of a sand cap, research attention turned toward the development of a surface material capable of bearing the weight of a sand cap.
Systematic tests were undertaken to develop a surface covering, natural or artificial, with a greater bearing strength. This would allow a greater depth and weight of sand to be placed on the clay wastes. Japanese millet was planted in one test. It thrived and within 10 weeks had produced a thick mass, 2 ft high with root systems extending 1½ ft down, which took over the entire pit and naturally reseeded itself.
The use of a flexible fiber covering (Du Pont Typar spun woven fabric) as a base for the sand cap was also tested. Problems developed when the flexible hoses pumping the sand wore through the Typar fabric and the sand was deposited below the surface. In another area where the concentration of the sand tailings was too high, the Typar fabric failed and the sand tailings passed into the clay. An attempt was made to place a Typar mat on the surface of the clay wastes and then to cover it with sand tailings from one side, progressing across the pit by the addition of new strips of Typar. This failed also because the sand tailings remained concentrated instead of flowing across the Typar surface. As they sank, the concentrated sand tailings pushed the fluid clay wastes out as a mud wave.
Another test pit was filled with a sand and clay slurry with a 3:2 sand-to-clay ratio and with an average solids content of 26.9%. The pit was covered with a growth of cattails, except for one small portion where Japanese millet was planted, which soon covered that area. A Typar strip was laid down near the edge and covered with sand tailings. A small bulldozer worked the tailings into a thin (2 ft) layer covering a wider area. Typar strips were then added, and more sand tailings deposited, to be worked into a thin layer by the bulldozer, working from the sides of the pit. This finally produced a 6-ft high mud wave of thickened clay in the pit center. But this too was successfully covered by the Typar and sand tailings. After the 2-ft sand tailings cap was completed, additional sand tailings were added to produce a sand cap averaging 6 to 8 ft in thickness. Once the sand cap was in place, additional dewatering and consolidation of the clay wastes continued- The tests achieved the expected results but seem unlikely to result in a workable, industrial-scale process because of the high equipment and labor costs.
Typar fabric is also being used in another test. High-ratio (4.9 parts sand to 1 part clay) sand-clay mix was pumped as a slurry onto the Typar mat. The mixture was easily and accurately delivered in a uniform sand cap without any failure of the Typar fabric. Corrugated metal sheets were placed below each outlet to minimize the impact of the incoming slurry upon the slurry that was already settling. Underneath the cap, the desired consolidation is progressing.
The research is continuing. There are questions of to what extent laboratory results can be duplicated in the field. In particular, there are questions of whether the effective stress of the sand cap will be dissipated through the expected 20- to 40-ft (or more) depth of the clay wastes and how far water can move through the clay. The IMC-Agrico- Mobil research project is still in progress. Tests are ongoing on a pit containing 27,000 tons of clay and 40,500 tons of sand that has recently been capped with 7 ft of tailings; a second pit has only clay wastes, 47,250 tons, at 21% solids content, 40 ft deep, but it has not yet been capped; a third shallow pit contains clay wastes at 18% solids content, covered with a Typar mat and capped with 1½ ft of sand and clay In the ratio of 4.9:1.
The greatest advantage of the dredge process is that it has the ability to dispose of both the sand and clay tailings in the same disposal site and to create a relatively stable landfill at the same time. The resulting surface is fertile for revegetation and agricultural purposes. The greatest disadvantage lies in the logistics difficulty of providing the needed ratio of sand and clay at the time they are required. This ratio normally depends upon the material in the matrix being mined and is not subject to easy adjustment by mine operators.
Sand Spray Process
Sand spray reclamation techniques grew out of early (1969 and before) tests of sand-clay mix disposal experiments. It was found, at the Brewster plant of American Cyanamid Co. (now Brewster Phosphates), that sand-clay mixes showed separation of the sand and clay when the mixtures were pumped into a settling pond. The sand fraction settled quickly while the clay fraction migrated and only settled slowly. It was found, however, that the sinking sand fell “gently” on thickened clay already in the pit. The sand migrating through the clay liberated large quantities of water and left behind clay solids that, over a period of time, approached 35% solids. This led to the concept of sand capping.
After small-scale experiments proved successful, large-scale tests of sand spray techniques were tested in 1974 and 1975. Figure 8 shows a flowsheet of the process. At Brewster’s Haynsworth Mine, five large cuts, typically 350 ft wide, 30 to 40 ft deep, and 2,000 ft long, were used for tests. Phosphatic clay slurries were pumped into the cuts and allowed to settle until they had reached approximately 13% solids. Then they were sprayed with sand from plant tailings. The sand was applied through a floating pipeline fitted with spray nozzles. The entire apparatus was movable to allow accurate and even placement of the sand over the clay wastes. The sand was sprayed on the clay at a rate of 825
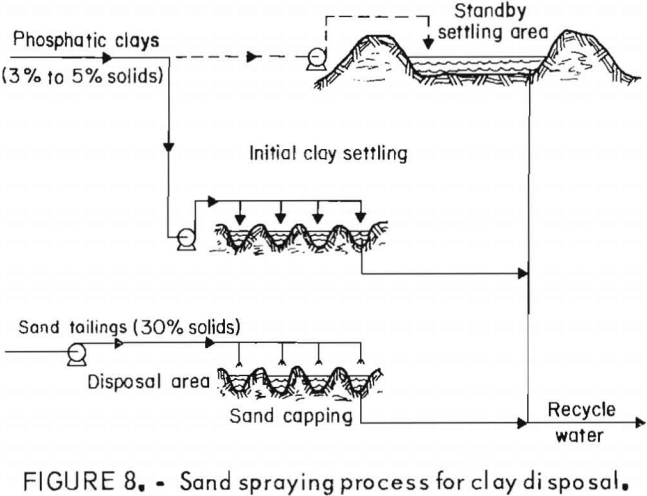
ton/h. Two separate passes of the sand spray were made, and each resulted in “significant” dewatering. The total solids content (for two passes) rose from 13% to 60%, and for the fine clay fraction (minus 150 mesh), it rose from 13% to 25%.
Occidental Chemical Co., under an FPCRP program, tested sand spray techniques at a 5-acre test site located at the White Springs Mine in 1974 and 1975. Approximately 20,500 tons of waste clay solids in the test pit had been sand-capped in August and September 1974. An additional 4,500 tons of clay waste averaging 12.2% solids were added. The overall solids content was approximately 21%. In a period of 4.5 h, some 4,300 tons of sand were sprayed over the settled clay-sand already in the pit. It was found impossible to add sand beyond a ratio of 1:5, sand to phosphatic clay. When this was exceeded, large-scale mud waving occurred. It proved difficult to spray the sand uniformly on the clay surface. The uneven weight caused the surface to heave. The sand spray test results were disappointing; the clay wastes in the top, recently added layer, increased from 12.2% to 15.8%, but the overall value for the fine clay material (minus 150 mesh) for the entire test pit remained unchanged. It was concluded that the “technique was not feasible under the conditions tested”. On the other hand, boreholes made 2 yr later revealed that the clays had continued to lose water and had reached 30% to 40% solids. The total solids content (sand and clay) ranged from 50% to 70%. This left a soil that provided sufficient strength for agricultural purposes (animals and vehicles). High pore pressures were also encountered, indicating that further dewatering could be expected through time.
Developing Technology
Gardinier Inc. Process
Gardinier, Inc., undertook tests from 1976 to 1979 of small pilot units of clay thickeners manufactured by six different companies. With equipment produced by the French company, Alsthom Atlantic, a sludge-bed-type, clay waste thickener pilot plant was designed and built. It was operated in 1980 and 1981. Gardinier and Alsthom Atlantic found that the (then) state-of-the-art flocculating and thickening pilot plant “did not increase dewatering of (clay wastes) beyond that which could be obtained by settling in ponds”.
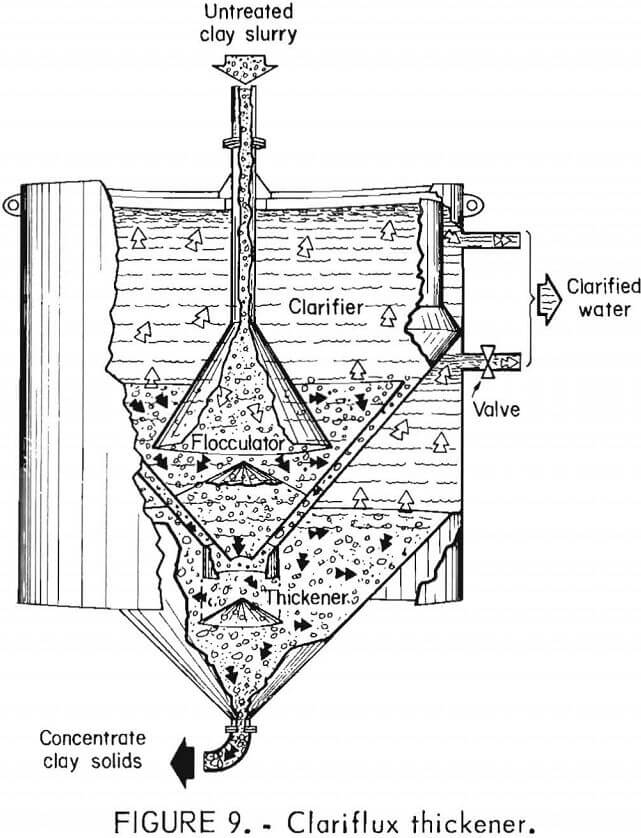
Further research by Gardinier and Alsthom Atlantic resulted in the development of a dewatering process for phosphatic clays called “superflocculation” or “aqueous agglomeration” or “pellet flocculation.” While the details have not been publicly released, it appears that the Gardinier process is essentially a two-stage flocculation and thickening process centered on Alsthom Atlantic’s Clariflux thickener.
The Clariflux unit acts as a flocculator, a clarifier, and a thickener. It is an entirely static unit utilizing an active sludge bed to concentrate floes. Untreated feed passes into the center of the unit, as shown in figure 9, where it is flocculated. Large flocs pass downward through an orifice into the thickener. The remainder moves upward into the clarifier unit with decreasing velocity into an active sludge bed. The slowing, small flocs agglutinate with the larger flocs already present. Size and density of the floes increase, the enlarged floes pass downward to the thickener, and clarified water moves upward where it is removed. The thickener is funnel shaped; the concentrated sludge is removed at a bottom outlet at a controlled rate to maintain the desired density. The flocculant used is a common one (unnamed), which Gardinier intends to manufacture itself.
Gardinier is building a large-scale test unit utilizing its process. Costing over $20 million, the system reportedly will handle all of the phosphatic clays generated by Gardinier’s Ft. Meade, FL, mine. It was scheduled to begin service in June 1983.
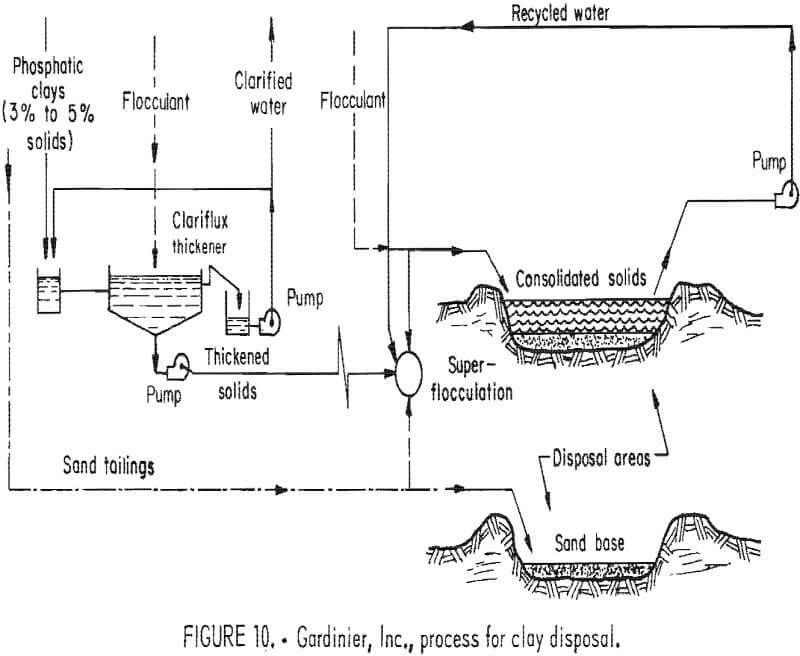
The test unit will take the phosphatic clay slurries from the phosphate plant averaging 1% to 6% clay solids content and pump them to five huge Clariflux thickeners (fig. 10). There the clays will be flocculated and thickened. The thickened clay underflow comprising 12% to 15% solids will be mixed with sand tailings and pumped to the previously mined-out cuts. Here will be located a “superflocculation station” where additional flocculant will be added. Large flocs form at this stage and when emplaced in the mining cuts reportedly dewater rapidly to near 25% solids in 24 h. The dewatering continues in the pits, and a solids content of 30% to 40% is reached in a few weeks, as reported by Gardinier. After this dewatering , more thickened clay-sand tailings will be placed in the pit in stages, to allow each addition to dewater before more is added. After approximately 1 yr, the sand-clay mixture in the pits will be capped with additional sand tailings and overburden. Gardinier anticipates that this will restore the mined-out areas to approximately the original contour before mining began. The entire process reportedly will require only 3 to 5 yr.
Gardinier believes that its process represents the “best available technology”. It will restore the land to pre-mining agricultural uses as soon as possible and presents a method of disposing of clay wastes below grade. This eliminates the danger of flooding and pollution from dam breaks. It also quickly frees large quantities of water, which will result, Gardinier believes, through better water management, in lower usage of water from the ground, thereby leaving more for other competitive uses.
Gardinier does not claim to have found the answer to the clay disposal problem; it claims only to have found the answer to its clay disposal problem. Gardinier points out that the process may not work for different ores from different mines and recommends that “extensive” investigations be undertaken before attempts are made to apply the Gardinier process to other ores and other conditions. Each situation may prove to be unique. Nevertheless, Gardinier is so confident of the success of its process that it is risking an investment larger than the total original 1967 cost of the entire mine, equipment, and processing plant on its outcome.
The greatest advantage of the Gardinier process is the rapidity with which the clay wastes can be dewatered and the consequent result that the total volume can be stored at or near ground level, eliminating storage ponds. It has, however, not been tested on a full industrial scale.
Bureau of Mines Rotary Trommel
The Bureau, as part of the FPCRP, undertook after 1972 the systematic testing of several types of reagents for possible use as clay settling agents. Nitrogen-bearing, sulfur-bearing, inorganic, and many commercially available reagents were tested for their ability to precipitate suspended clay particles. The reagents were tested in the laboratory against a representative cross section of phosphatic clay slurries from several different mines in central and northern Florida. The reagents were also tested at varying concentrations (0.1, 0.01, and 0.001 M) and at varying pH levels. Neither the reagents, their concentration, nor the pH of the slurries had any significant effect upon the clay suspensions, with two exceptions.
Hydrofluoric acid (HF) caused the suspended clay particles to settle out vigorously, particularly the attapulgite clay fraction. Other fluorine compounds, such as hydrofluosilicic acid and ammonium fluoride, also caused the suspended clay to precipitate. However, because of expense and environmental considerations, the use of fluorine compounds was not considered feasible for treating clay discharge.
One commercially available reagent caused an unusually strong flocculation and dewatering effect in attapulgite samples and even a stronger reaction with montmorillonite and other plant phosphatic clays. The reagent was polyethylene oxide (PEO), a water-soluble, non- ionic polymer with a molecular weight of 5 to 8 million. PEO has the approximate formula (CH2-CH2-O)x.
Because of its promise, further tests were run on the PEO-clay interaction. It was found that there was no correlation between the composition of the clay slurry and the PEO needed to dewater it. Each clay sample had to be evaluated individually, and the samples exhibited a wide range of consumption of PEO when dewatered. Laboratory tests were also undertaken to determine the effects of PEO concentration on five samples of mine clay wastes. The concentrations tested were 0.25%, 0.10%, 0.05%, 0.01%, 0.005%, 0.0025%, and 0.001%. It was found, generally, that lower concentrations required the use of less reagent, but at extremely low concentrations, so much more water was being added to the slurry that it made the dewatering even more difficult. Tests were also carried out to determine the effect of varying solids content in the phosphatic clay feed. Generally, it was seen that an increased amount of reagent was required with an increased percent solids content of the clay slurry feed.
Experiments were also done to create a continuous process using PEO. A horizontal vibrating screen dressed with 100- mesh wire cloth was tested and successfully dewatered the clay waste-PEO slurry to 29% solids. A curved, static, wedge-wire screen was used to dewater a 3.9% solids phosphatic clay feed to 17% solids. This in turn was dewatered further in tests, to 31% using a horizontal vibrating screen and to 27% using a rotating trommel. A screw classifier successfully dewatered 3.9% solids clay slurry to 30% solids. A rotating trommel (0.38 m in diam, 0.9 m in length) fitted with 10-mesh wire screen was also used in tests. In the trommel, the clay-PEO slurry, previously mixed, dewatered very rapidly and formed consolidated rolls or masses, as it moved through the trommel, of 25% to 30% solids phosphatic clay. The discharge water, however, contained some flocculated solids that leaked through the first 25 to 30 cm of the trommel screen.
Other small-scale tests investigated the effects of such variables as trommel speed, trommel slope, reagent concentration and dosage, retention time, feed rate, and variations in clay slurry feed and reagent mixing.
The success of small-scale dewatering of phosphatic clays with PEO and a rotary trommel led to tests on a larger scale. A field test unit, designed to treat 100 gal/min of dilute phosphatic clays, was fabricated and operated at Estech General Chemicals Corp.’s Silver City Mine (fig. 11). The trommel screen of the field test unit was built of 10-mesh stainless steel wire screen. It was 24 ft long and 3 ft in diam. Later the trommel was shortened to 12 ft, and a static screen or hydrosieve was added. The first 6 ft of the feed end of the rotary trommel was also lined with 48-mesh stainless steel screen to prevent premature loss of fine particles of agglomerate. The clay slurries were pumped into a mixer at rates from 60 to 124 gal/min and then into the trommel, or into the hydrosieve screen and then into the trommel. The dewatered clays exited the trommel at 14% to 20% solids and were pumped to a pit where dewatering continued.
The large-scale tests were carried on intermittently over an 18-month period. Wide variability in the nature of the waste clay feed was soon observed. Plant operators reported that a fringe area was being mined and that the mined material was not typical of normal phosphate deposits in the area. The variability of the clay waste composition caused many problems for operations of the field test unit. Lime had to be added to several samples to oxidize the noncalcium exchanged clay.
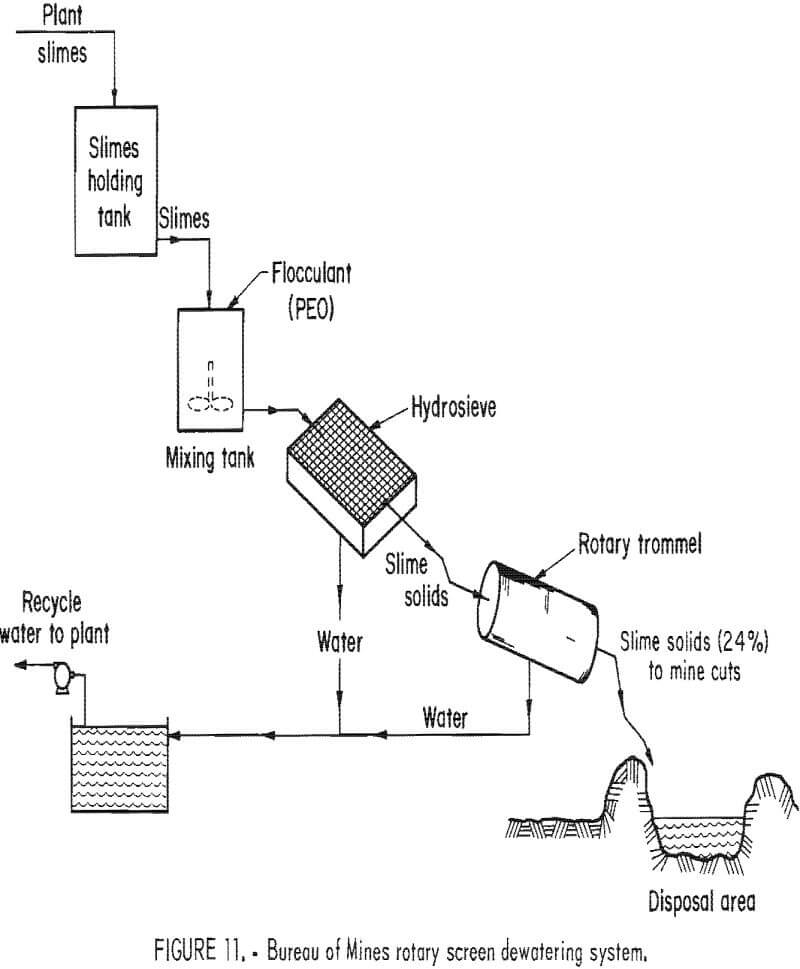
Results of the large-scale test unit were satisfactory. The solids content of the dewatered clay slurries ranged from 14.3% to 16.0% for a reagent usage of 3.0 lb/ton of PEO at 0.25% concentration to 1.3 lb/ton PEO at 0.025%. When the PEO dosage was increased, it did not produce significantly higher solids content in the dewatered clay wastes. PEO dosages as high as 6 lb/ton improved the solids content to only about 16.2%.
The Silver City Mine clays were effectively dewatered with lower reagent usage by using PEO of about 8 million mol wt. This is not necessarily true for any other mine phosphatic clays. Using very dilute PEO (0.0025% concentration) allowed the dosage to be reduced to 0.5 lb/ ton. But the additional water deleteriously affected the efficiency of the trommel. The hydrosieve screen was placed between the mixer and the trommel to overcome this problem. Clay slurries at 3.0% solids were dewatered by the hydrosieve screen to about 8,8% solids before being introduced into the trommel.
The field test unit operating at 100 gal/min, using 0.01% and 0.005% concentrations of PEO, dewatered phosphatic clay slurries to 20.6% solids with a consumption of 0.69 lb of PEO reagent per ton. It was also necessary to use 1 or 2 lb of lime per ton.
The dewatered clays were then pumped into a pit 15 by 45 by 4 ft deep at 16% to 20% solids to test continued dewatering, Within 30 days, the clays had dewatered to 30% solids; after 45 days, to 40% solids. This continued dewatering came despite the fact that there was a rainfall of 18 in during the first 30 days the clay samples were in the pit.
Further laboratory-scale studies were undertaken by the Bureau to determine if cheaper substitutes could be found for PEO. In particular, several groups of reagents were investigated for possible synergistic effects when used with PEO. Most were found to be of little use; only those capable of flocculating the phosphatic clays proved useful. One class of reagents, natural guar gums, did produce a synergistic effect with PEO, which resulted in reducing the amount of PEO required by 54%.
Summary
The disposal of phosphatic clays has been a problem to the Florida phosphate industry that has grown with the industry and today represents probably one of the mining industry’s largest waste-handling problems. Although large clay storage impoundments have been the most practical solution, they are unpleasing aesthetically, present a possibility of dam failure, and require considerable acreage that could be used for other purposes. Immense amounts of water are tied up in the impounded clays that are desired for alternative uses.
Recognizing these problems long before the age of environmental awareness, the phosphate companies have almost continually sought answers to the problem of clay dewatering. Millions of dollars and uncountable hours have been spent by the industry, the Bureau of Mines, and private and other governmental researchers on the phosphatic clays problem. The companies have vast amounts of money tied up in the real estate used for storage areas and in the need for replacement water used in mining and beneficiation.
All of this work has resulted in the numerous systems described in this report. Many methods to dewater the clays resulted from these studies. However, their technical feasibility has not been fully demonstrated; therefore, they have not found full-scale adoption in the industry. The most common drawbacks have been of cost, either prohibitively high original investment costs or operating costs, particularly energy costs. There were also numerous problems of scale. Processes that showed promise in the laboratory often failed on a large scale. New technical problems were created by some of the experimental dewatering techniques, for example, when flocculants were added to the clays. But the most significant obstacle to the development of any universally effective dewatering process was the extreme variability of the clays themselves. Because of their widely variable nature throughout the phosphate district and even within one mine, successful methods used to dewater one suite of clays failed tests on another. Dewatering processes were found to be highly site specific.
While many of the experimental and developing dewatering techniques show promise of future success and some may even be practical now under radically changed economic conditions, none can presently replace conventional settling in terms of cost and efficiency. Several of the developing techniques described are already being incorporated into conventional processes where they are used along with conventional settling processes to speed the rate of initial dewatering.
