Studies of sulfidization and flotation of chrysocolla involving the use of pH and sulfide ion instruments, a Zeta meter, microflotation techniques, and sensitive determinations of adsorbed sulfur have given new insight into the mechanism of the process.
Factors that affect the recovery of sulfidized chrysocolla by flotation appear to be the pH at which it is sulfidized, and certain alterations of the surface that occur during aging after sulfidization. Such aging results in increased recoveries.
After some preliminary experimentation, the following procedure was adopted for the sulfidization step in all experiments. Fresh solution was prepared for each series of three sulfidizations by dissolving a weighed quantity of 63 percent, fused sodium sulfide chips in distilled water, and diluting to one liter. Then 300 ml of this solution was placed in a beaker on a magnetic stirring plate, and electrodes for the determination of sulfide ion activity and pH were submerged in it. The pH was adjusted using hydrochloric acid, and it was maintained within a few thousandths of the desired value during the sulfidization by dropwise addition of either more HCl or more of the sodium sulfide solution. Acid was required during the first half while the basic sulfide solution was required during the second half of each sulfidization to maintain constant pH values.
In dilute sulfide solutions the concentrations of H2S, HS- ion, and S= ion may be calculated from the total sulfide content and the pH by means of ionization constants. In addition to doing this, the activity of sulfide ion was monitored before and during each sulfidization by means of an Orion sulfide ion activity electrode and an expanded scale pH meter. In this way it was determined that the variations in sulfide ion activity during sulfidization of each sample were no greater than occur in similar solutions without chrysocolla.
Two series of tests were made in which the variables were the conditions under which the samples were sulfidized, these conditions being within a pH range from 4.0 to 6.2 and a range of concentration from 250 to 700 mg of 63 percent Na2S per liter. These conditions cover the island of collectibility reported by Bowdish and Chen in the first or non-aged series the chrysocolla was placed in the flotation cell immediately after it had been sulfidized and washed; in the second or dry-aged series the samples were dried for 25 minutes at 40°C before being floated.
Chrysocolla could not be floated immediately after sulfidization regardless of the sulfidizing conditions. Flotation recoveries in the twenty-four non-aged tests ranged from 0.63 to 11.8 percent with an average of 5.43 percent. Statistically, at the 95 percent confidence level all the recoveries may be considered equal at the average. In a similar flotation test on an unsulfidized sample the recovery was 4.31 percent.
After the flotation failures of the series of non-aged tests, the dry-aged tests were made because during preliminary testing of the microflotation cell good floatability had been observed with chrysocolla that had been sulfidized and dried.
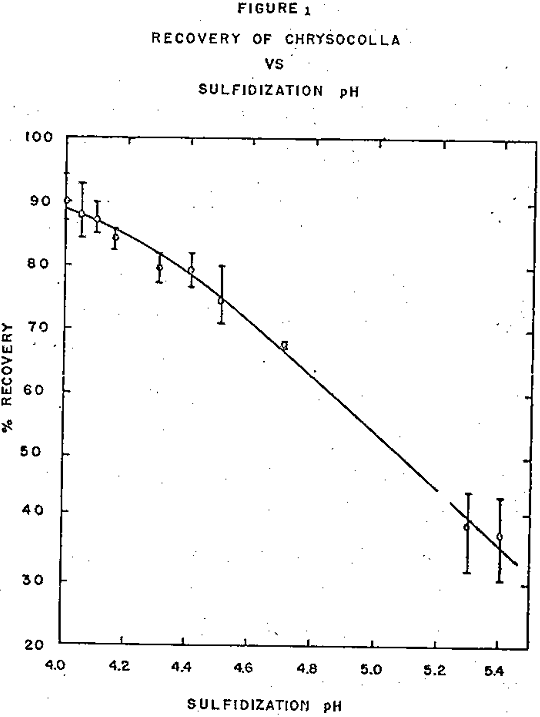
However, the recovery was strongly dependent upon the pH of sulfidization. As this pH was lowered from 5.4 to 4.0 the recovery increased steadily from about 35 to about 90 percent. Unfortunately, no data are available for pH values below 4.00 but it is expected that flotation of chrysocolla would decrease rapidly at pH values below that at which the copper becomes soluble, or roughly below pH 4.0.