Table of Contents
- Materials and Test Methods
- Reagents
- Results
- Contact Angle
- Anionic Surfactants
- Sodium Oleate
- Sodium Isooctyl Phosphate
- Sodium Heptadecyl Sulfate
- Sodium Dodecyl Benzene Sulfonate
- Sodium Dioctyl Sulfosuccinate
- Fatty Acid Amide
- Cationic Surfactants
- Primary Amines
- Other Cationic Surfactants
- Amphoteric and Nonionic Surfactants
- Microflotation
- Anionic Surfactants
- Sodium Oleate
- Sodium Isooctyl Phosphate
- Sodium Heptadecyl Sulfate
- Sodium Alkyl Sulfonate
- Sodium Dioctyl Sulfosuccinate
- Cationic Surfactants
- Primary Amines
- Diamine
- Tertiary Alkyl Amine
- Quaternary Amine
- In contact-angle research, 10 of the 17 surfactants evaluated proved to be collectors for Chrysoberyl Flotation.
- With eight of the nine surfactants evaluated as collectors, microflotation recoveries of beryl, feldspar, and topaz were equal to or greater than recoveries of chrysoberyl.
- Ore-gangue mineral selectivity of all collectors evaluated, except isooctyl phosphate, will be low.
- Further study of collectors of the alkyl-phosphate type for beryl and chrysoberyl flotation is warranted.
- Seven collectors showed promise of simultaneous flotation of both beryl and chrysoberyl.
- Further study directed toward evaluation of chrysoberyl activation and gangue-mineral flotation and depression is warranted.
Improved beneficiation of beryllium ores is needed to meet future requirements for beryllium. These beryllium requirements are increasing not only because of expansion of current uses but because of new and significant applications developed by research in nuclear energy, aerospace, and space exploration. This investigation is a part of the program to develop and utilize domestic beryllium resources. Its purpose is to provide basic information to aid subsequent flotation research for commercial flotation of specific ores.
In this research both contact-angle and microflotation methods were used. Major emphasis was directed toward chrysoberyl because, although significant deposits of the mineral are known, only meager information is available concerning its flotation characteristics. As a complement to the research on beryllium minerals, the flotation response of some gangue minerals common to beryl and chrysoberyl deposits was studied by microflotation.
The majority of the earlier beryllium mineral flotation research was concerned with the flotation of beryl with fatty acid collectors. Recently, however, some studies of other reagents such as sulfonates have been made. Successful continuous-circuit flotation of bertrandite and phenacite with fatty acids has been reported. There is general correlation between information developed in this study and that found in related prior literature. Specific agreement was found between the results of this study and those of others reporting the use of alkyl sulfate (8).
Materials and Test Methods
Materials
Minerals
Beryllium minerals studied by contact-angle methods were beryl, chrysoberyl, and phenacite. Only beryl and chrysoberyl were studied by microflotation, but the associated gangue minerals, calcite, feldspar (microcline), fluorite, quartz, and topaz were included. Specimens for contact-angle studies were selected for mineral purity and freedom from fractures. Minerals for microflotation tests were selected for mineral purity and freedom from surface alteration. Mineral specimens were stored under distilled water to avoid surface contamination. Three of the most significant ore minerals- beryl, chrysoberyl, and phenacite-were obtained in a form suitable for contact-angle study. Two of those minerals, beryl and chrysoberyl, were obtained in sufficient quantity and purity necessary for microflotation tests.
Reagents
Reagents used in this study were: (1) Surfactants which were either high-purity commercial products, or were prepared from them with reagent-grade acids or bases; (2) common acids, bases, and inorganic salts of reagent grade; and (3) other high-grade industrial compounds such as tapioca starch, dextrin, and calcium lignin sulfonate. Distilled water was used to make up reagent and test solutions and to rinse the glass apparatus after it had been cleaned with sulfuric-chromic acid solution.
Surfactants studied are classified in three groups for convenience: Anionic, cationic, and others. In the third group are amphoteric and nonionic surfactants. A list of the 17 individual surfactants tested in the contact-angle study is presented in table 1 together with a tabulated summary of contact-angle results. (Those reagents that also were used in the microflotation studies are noted in table 1.) The solution concentrations used in this report represent the concentration of the active compound, or groups of active compounds, as closely as could be determined from information supplied by the producers.
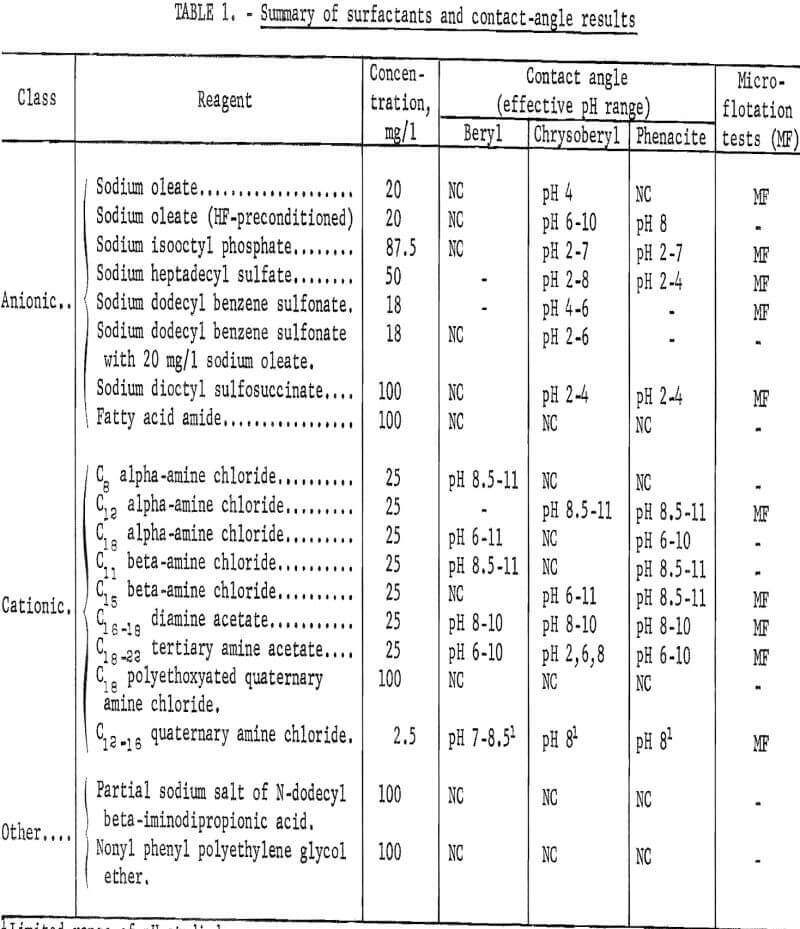
Further information concerning individual reagents and their specific application is given at the sections in which contact-angle and microflotation results are reported.
Test Methods
Apparatus and procedures used for both contact-angle and microflotation research were similar to those used by other investigators and are described in detail in Report of Investigation 7189, Microflotation Studies of Some Columbium-Tantalum Minerals.
For contact-angle studies, a modified free-bubble method was used. Standard time for specimen conditioning was 5 minutes. In both contact-angle and microflotation studies, the pH of test solutions was controlled by additions of NaOH and H2SO4, except for a few tests in which HCl or HF were used. Therefore, specific mention of the acid used is made only when H2SO4 was not employed. For microflotation research a modified Hallimond tube was used that was similar in design to that used by Iwasaki. Mineral samples of approximately 1 gram in weight were conditioned by shaking for 5 minutes at the pre-determined test pH, were transferred to the Hallimond tube, and were aerated for 5 minutes with a measured flow of water-pumped nitrogen. The volume of the test solution was 140 ml. Conditioning and flotation time of 5 minutes was selected on the basis of contact-angle and preliminary microflotation tests as sufficient. No study was made of optimum conditioning or flotation time.
Three qualitative terms, “no contact,” “cling,” and “threshold” are used in this report to supplement the numerical data and to aid in defining ob served conditions.
The first term, “no contact,” abbreviated NC, denotes bubble-mineral attraction is nil; the second term, “cling,” is used to describe the condition of bubble-mineral attraction great enough to distort the bubble slightly but without indication of possible bubble attachment; “threshold,” abbreviated Thres, denotes a condition of bubble attraction significantly greater than “cling,” but one not resulting in ready bubble attachment.
Results
Contact Angle
Significant contact angles were observed in solutions of 12 surfactants. A list of surfactants tested and a brief summary of all of the contact-angle tests are given in table 1.
Anionic Surfactants
Contact angles on three beryllium minerals were studied in solutions of six anionic surfactants: Sodium oleate, sodium isooctyl phosphate, sodium heptadecyl sulfate, sodium dodecyl benzene sulfonate, sodium dioctyl sulfo- succinate, and an anionic-active fatty amide derivative.
Purity and preparation of anionic surfactants was varied. The sodium oleate used was a purified neutral salt. Sodium isooctyl phosphate was prepared by neutralization of acid isooctyl phosphate. Sodium heptadecyl sulfate was supplied as a 25-percent aqueous solution. Sodium dodecyl benzene sulfonate as supplied contained less than 10 percent Na2SO4 as the sole significant impurity. Sodium dioctyl sulfosuccinate was supplied as a 60-percent solution in a mixture of equal parts of isopropanol and water.
The fatty acid amide tested is reportedly an anionic-active fatty acid amine derivative. It was supplied as an aqueous paste containing 40 percent solids. Neither the purity of the contained solids nor the molecular weight of parent fatty acids could be determined from available information; however, other data given by the manufacturer indicates they are of high molecular weight.
Sodium Oleate
Three beryllium minerals, beryl, chrysoberyl, and phenacite, were tested in solutions containing 20 mg per liter of sodium oleate with and without 5-minute preconditioning in 10 percent hydrofluoric acid solution. Hydrofluoric acid of this strength was used to insure clean mineral surfaces. The pH ranges investigated were 2 to 11 with normal conditioning and 4 to 11 with HF-preconditioning. No contact angles were observed on beryl. On phenacite the only contact angles observed were with HF-preconditioning at pH 9 (41 degrees) . Chrysoberyl was more responsive; for instance, with no HF-preconditioning a significant contact angle was observed only at pH 4 and with HF-preconditioning significant contact angles were observed through a pH range of 4 to 10. Contact angle at pH 4 with HF-preconditioning (60 degrees) was more than 10 degrees higher than that found without HF-preconditioning. The highest contact angles (more than 70 degrees) were observed at pH 9 and 10.
Sodium Isooctyl Phosphate
The three beryllium ore minerals were tested in solutions containing 87.5 mg sodium isooctyl phosphate per liter through the pH range 2 to 11. Significant contact angles (46 to 54 degrees) were observed on chrysoberyl and phenacite only at pH levels 2 to 7. No contact angles were observed on beryl.
Sodium Heptadecyl Sulfate
Observations of contact angles on beryl, chrysoberyl, and phenacite were made in solutions containing 50 mg of sodium heptadecyl sulfate per liter. Hydrochloric acid and NaOH were used for pH control. Chrysoberyl and phenacite were tested through the range pH 2 to 10. These results, reported in table 2, show no contact at pH 10 and 11, with highest contact angles at low pH. Beryl was tested with sodium heptadecyl sulfate only at pH 10 and no contact was observed. Unpublished research with other alkyl sulfates suggests the contact-angle pattern for beryl is similar to that for chrysoberyl and phenacite.
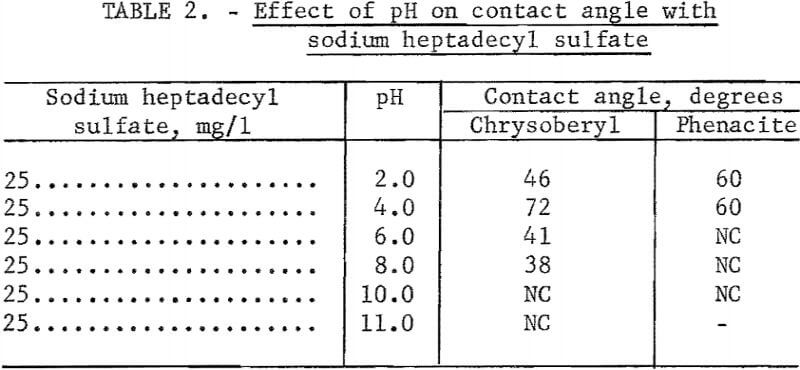
Note.-NC means “No contact.”
Sodium Dodecyl Benzene Sulfonate
The three beryllium minerals were tested with sodium dodecyl benzene sulfonate used alone and with sodium oleate. With 180 mg per liter sodium dodecyl benzene sulfonate, no bubble contact was observed through the pH range 2 to 8 on beryl, chrysoberyl, or phenacite. When chrysoberyl was tested through the pH range 2 to 12 with 90 mg per liter of sodium dodecyl benzene sulfonate only one contact angle was observed -60 degrees at pH 6. However, at a concentration of 20 mg per liter contact angles of 61 degrees at pH 4 and 82 degrees at pH 6 were observed on chrysoberyl.
Similar results were obtained on chrysoberyl when 20 mg per liter sodium dodecyl benzene sulfonate was used with equal weights of sodium oleate. Between pH 2 to 6 contact angles on chrysoberyl ranged from 60 to 70 degrees. However, no measurable contact angles were found on either phenacite or HF-preconditioned beryl in the pH range 2 to 11. At a solution strength of 100 mg per liter of each reagent the chrysoberyl contact angle at pH 5.5 was 65 degrees, but at pH there was no contact.
Sodium Dioctyl Sulfosuccinate
Each of the three beryllium minerals was tested in 100 mg per liter solution of sodium dioctyl sulfosuccinate through the pH range 2 to 10. Hydrochloric acid was used with sodium hydroxide for pH control, chrysoberyl and phenacite showed contact angles at pH 2 of 52 and 44 degrees and at pH 4 of 44 and 38 degrees, respectively. No contact was found for either of these two minerals at pH 6 or higher. No contact was found on beryl through the range tested, pH 2 to 10.
Fatty Acid Amide
In solutions of 250 mg per liter of the fatty acid amide, no contact was observed on the beryllium minerals. The ranges of pH investigated for each mineral were: chrysoberyl, pH 2 to 11; phenacite, pH 2 to 10; and beryl, pH 8 to 11.
Cationic Surfactants
Cationic surfactants were the most responsible group of surfactants studied. Included in this study were five primary amines, one diamine, one tertiary amine, and two quaternary amines. As was the case with the anionic surfactants, purity and preparation of the cationic surfactants varied. Except for the C16-18 diamine acetate the total amine content of these amine surfactants was essentially 100 percent. The principle amine (used to identify the reagent) accounted for more than 90 percent of the amine content of the primary alpha-amines. Amines with other carbon-chain lengths accounted for less than 1 percent of the total amine content of the C12 alpha-amine.
Primary alpha-amines used were: C12 alpha-amine (1-aminooctane); C12 alpha-amine (1 aminododecane) ; and C18 alpha-amine (1-aminooctadecane). Less information is available for the primary beta-amines; however, both primary beta-amines used were the highest quality available commercially. These products are designated by the manufacturer by the shortest carbon chain of the component beta-amines. This designation is also used in this report to identify the two beta-amines used which were C11 beta-amine (2-aminohendecane) and C15 beta-amine (2-aminopentadecane).
The purity of the C16-18 diamine acetate was more than 85 percent diamine acetate that was 95 to 100 percent neutralized.
The tertiary alkyl amine was neutralized to use it as the soluble acetate. The alkyl groups were principally those having carbon-chain lengths of C18-22.
Both quaternary amines used were water soluble. One was C18 polyoxylated quaternary amine chloride that contained two moles of ethylene oxide in its structure. It was used as received. The other quaternary amine reagent was a blend of three quaternary ammonium chloride salts differing only in alkyl groups: C12 40 percent; C, 14, 50 percent; and C16, 10 percent. Average molecular weight of the active amine was 358. This amine reagent is designated as C12-16 quaternary amine chloride and was supplied as a 50-percent solution in 40 percent water and 10 percent ethanol.
Primary Amines
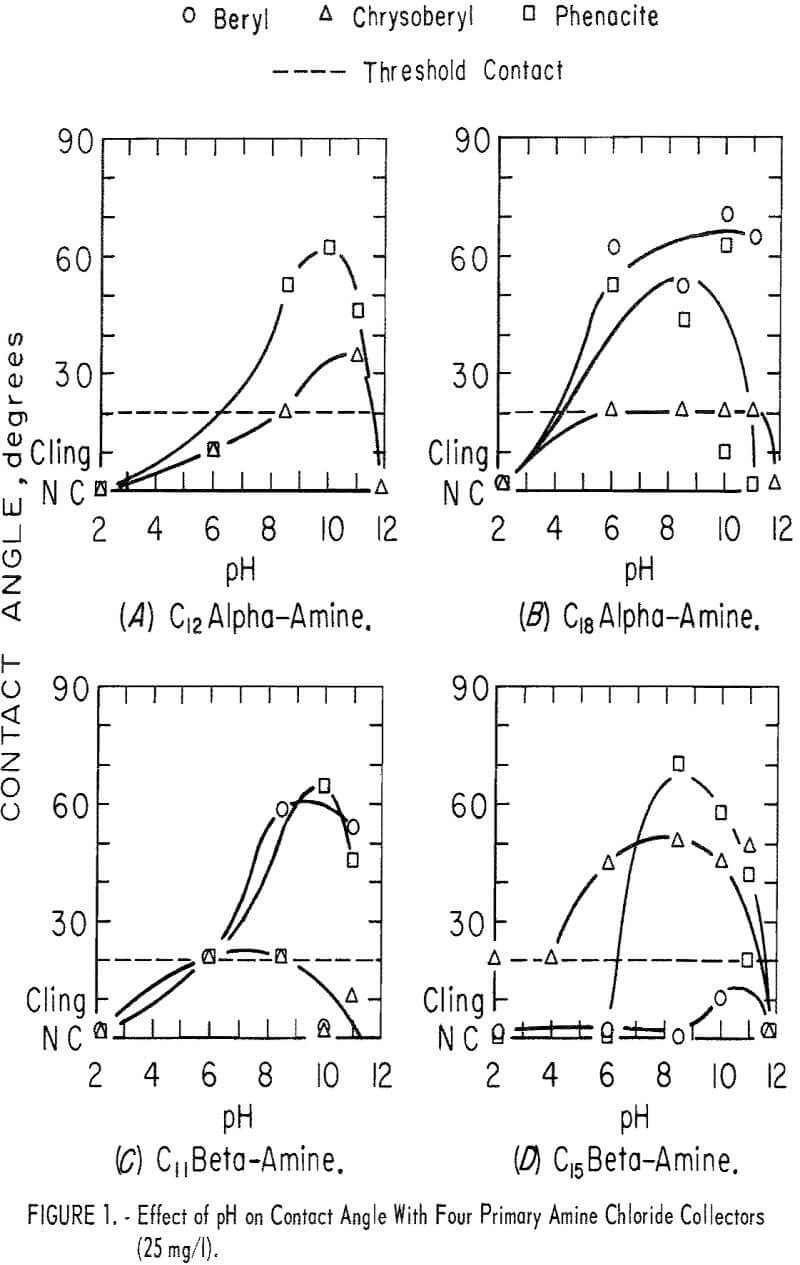
Five primary amines, three alpha-amines, and two beta-amines were used as the chloride salts in parallel contact-angle tests. In each of these tests the primary amine chloride concentration was 25 mg per liter, and HCl was used with NaOH for pH control. In most of the tests of this group the pH range investigated was pH 2 to 11. Contact angles on beryl and phenacite were highest in solutions of primary alpha-amines with the longest carbon chains. Significant contact angles were found on chrysoberyl only in solutions of the beta-amine with the longest carbon chain.
In solutions of C8 alpha-amine no contact was observed except in the range of pH 8.5 to 11. In this range threshold readings were obtained on chrysoberyl and phenacite. On beryl, contact angles were near 40 degrees. Data obtained with the other four primary amines are shown in figure 1. They show a strong collector action at moderately high pH levels.
Other Cationic Surfactants
Four other cationic reagents were tested: C16-18 diamine acetate; C18-22 tertiary amine acetate; C12-16 quaternary amine chloride; and C18 polyethoxylated quaternary amine chloride.
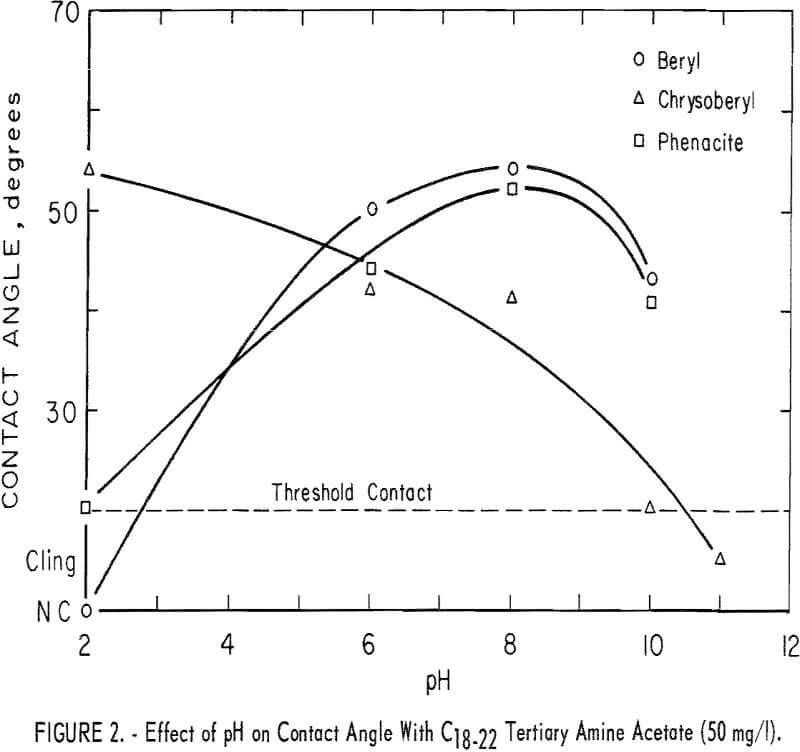
With the C16-18 diamine acetate in a concentration of 25 mg per liter, significant contact angles were observed on all three beryllium minerals in the range pH 8 to 10; none was observed at pH 2 or 4. Results with 50 mg per liter of C18-22 tertiary amine are shown in figure 2. Significant contact angles were observed on all minerals tested over a substantial pH range. Chrysoberyl response was greatest at pH 2, while response of beryl and phenacite was greatest at pH 8.
Limited tests with one of the two quaternary reagents, C12-16 quaternary amine chloride at 2.5 mg per liter concentration, showed it to be an effective collector at moderate pH levels. Contact angles ranging from 47 to 62 degrees were observed on beryl at pH 7 to 8,5, and on phenacite the contact angle was 43 degrees at pH 8. At pH 9 no contact was observed on either beryl or phenacite. The second quaternary amine, C18 polyethoxylated quaternary amine chloride, was used at a concentration of 100 mg per liter. No contact was observed on any of the three minerals tested in the pH range 2 to 10.
Amphoteric and Nonionic Surfactants
In contact-angle tests with either the amphoteric surfactant or with the nonionic surfactant no significant contact angles were observed on any of the three beryllium minerals through the pH range 2 to 10. Surfactant concentrations were 100 mg per liter. The amphoteric surfactant was a partial sodium
salt of N-dodecyl beta-iminodipropionic acid. The nonionic surfactant used was a nonyl phenol polyethylene glycol ether containing 10.5 moles of ethylene oxide in its structure.
Microflotation
Microflotation studies proved and extended the information obtained in the contact-angle research. Minerals studied included two beryllium minerals, beryl and chrysoberyl, and the gangue minerals calcite, feldspar, fluorite, quartz, and topaz, chrysoberyl received primary emphasis. Ten collectors, five anionic, and five cationic collectors, were studied in single mineral flotation, both with and without modifying reagents.
Modifiers used in the microflotation tests included four of reagent grade- ammonium carbonate, magnesium silicofluoride, aluminum sulfate, and sodium carbonate- and four of industrial grade -calcium lignin sulfonate, dextrin, tapioca starch, and sodium silicate. Calcium lignin sulfonate and dextrin were considered 100 percent reagents and were used as received. The tapioca starch was procured as Siamese tapioca flour and also was considered a 100-percent reagent. Sodium silicate of technical grade N, Baume 41, was diluted to give two concentrations (15 and 300 mg per liter) of actual sodium silicate.
Anionic Surfactants
Sodium Oleate
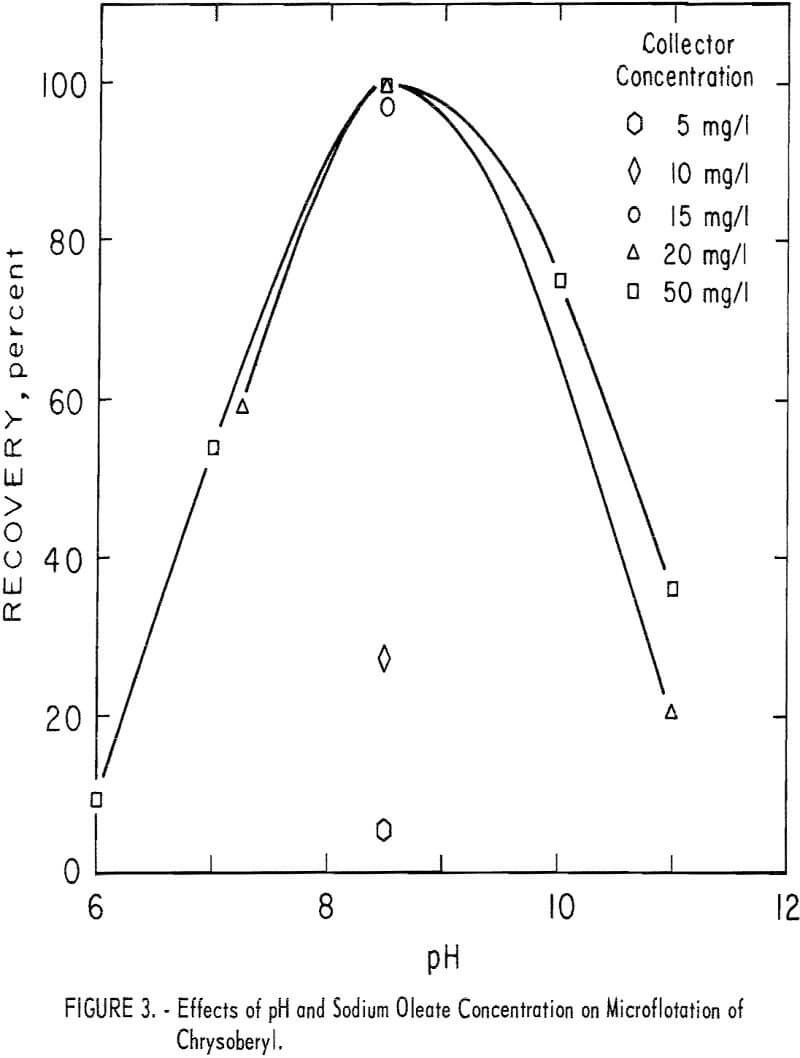
Microflotation recoveries of chrysoberyl were complete with 20 mg per liter sodium oleate at pH 8.5. Pertinent data at other pH and collector concentration levels are reported in figure 3 and show that a collector concentration between 15 and 20 mg per liter is necessary for complete recovery of chrysoberyl at pH levels close to pH 8.5.
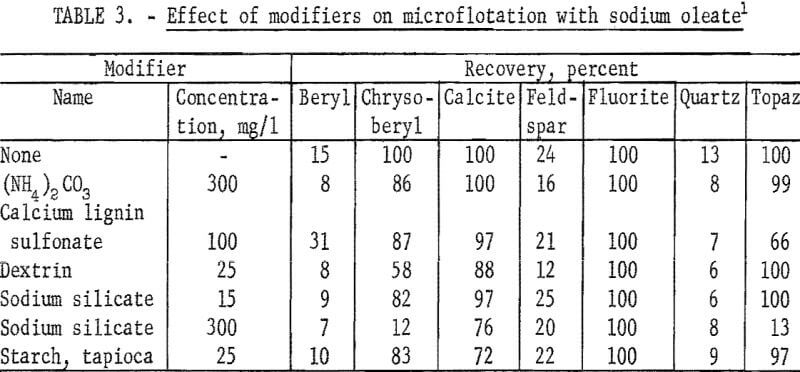
In table 3, microflotation data are reported for beryl, chrysoberyl, and five gangue minerals with 20 mg per liter sodium oleate at pH 8.5 alone and with each of six modifiers. These results show sodium oleate is not a selective collector for chrysoberyl or beryl. Calcite, fluorite, and topaz recoveries exceeded those of the two beryllium minerals for all but one condition.
Sodium Isooctyl Phosphate
Sodium isooctyl phosphate was a good collector for calcite, fluorite, and topaz, as well as for beryl and chrysoberyl. At pH levels below pH 2 differential flotation of chrysoberyl and beryl from quartz or fluorite should be possible. Collector concentrations investigated were 100, 50, and 25 mg per liter for chrysoberyl and 25 mg per liter for other minerals. At the pH levels 6, 7, and 8 test solutions were buffered with 100 mg NaHCO3 per liter.
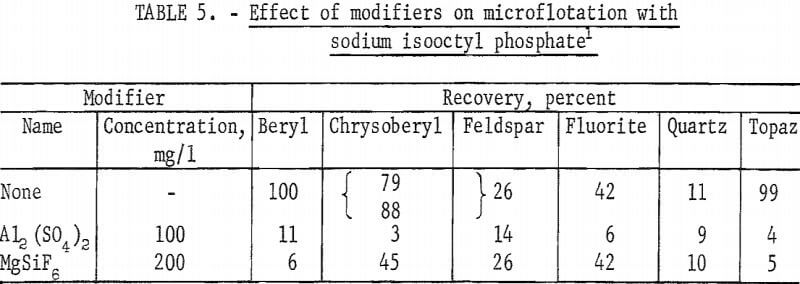
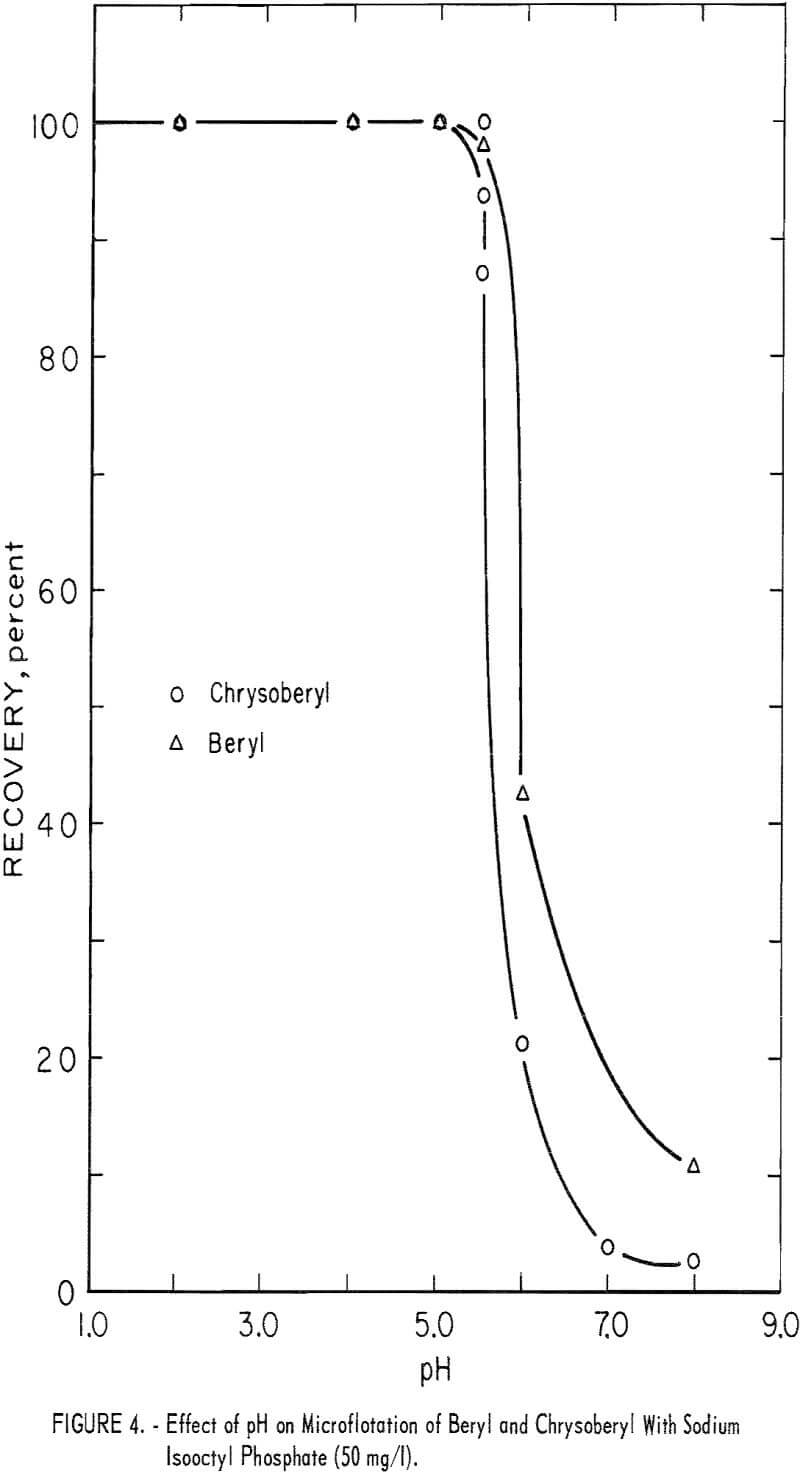
Chrysoberyl floated best in the pH range 4 to 5; recoveries were 100 percent with 25 mg sodium isooctyl phosphate per liter. At pH 6 chrysoberyl recovery dropped to 6 percent. As collector concentration increased, the pH range of high recovery broadened. Chrysoberyl recovery at 100 mg per liter was essentially complete through the range pH 1.5 to 6.0. At pH 4, with this amount of reagent, complete recovery was obtained in 10 seconds. In comparison, recoveries for 1-minute flotation with collector concentrations of 25 and 50 mg per liter were 82 and 100 percent, respectively. Five-minute recovery data presented in figure 4 show parallel chrysoberyl and beryl recoveries through a wide pH range.
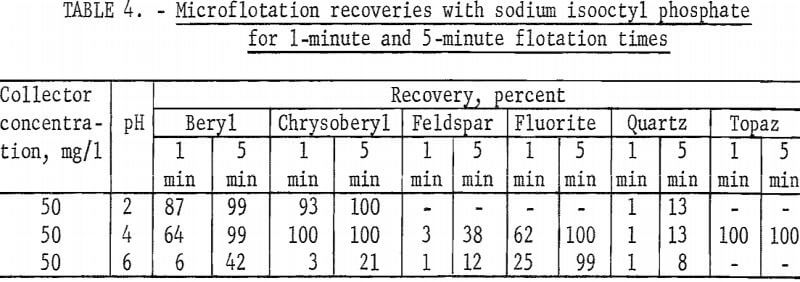
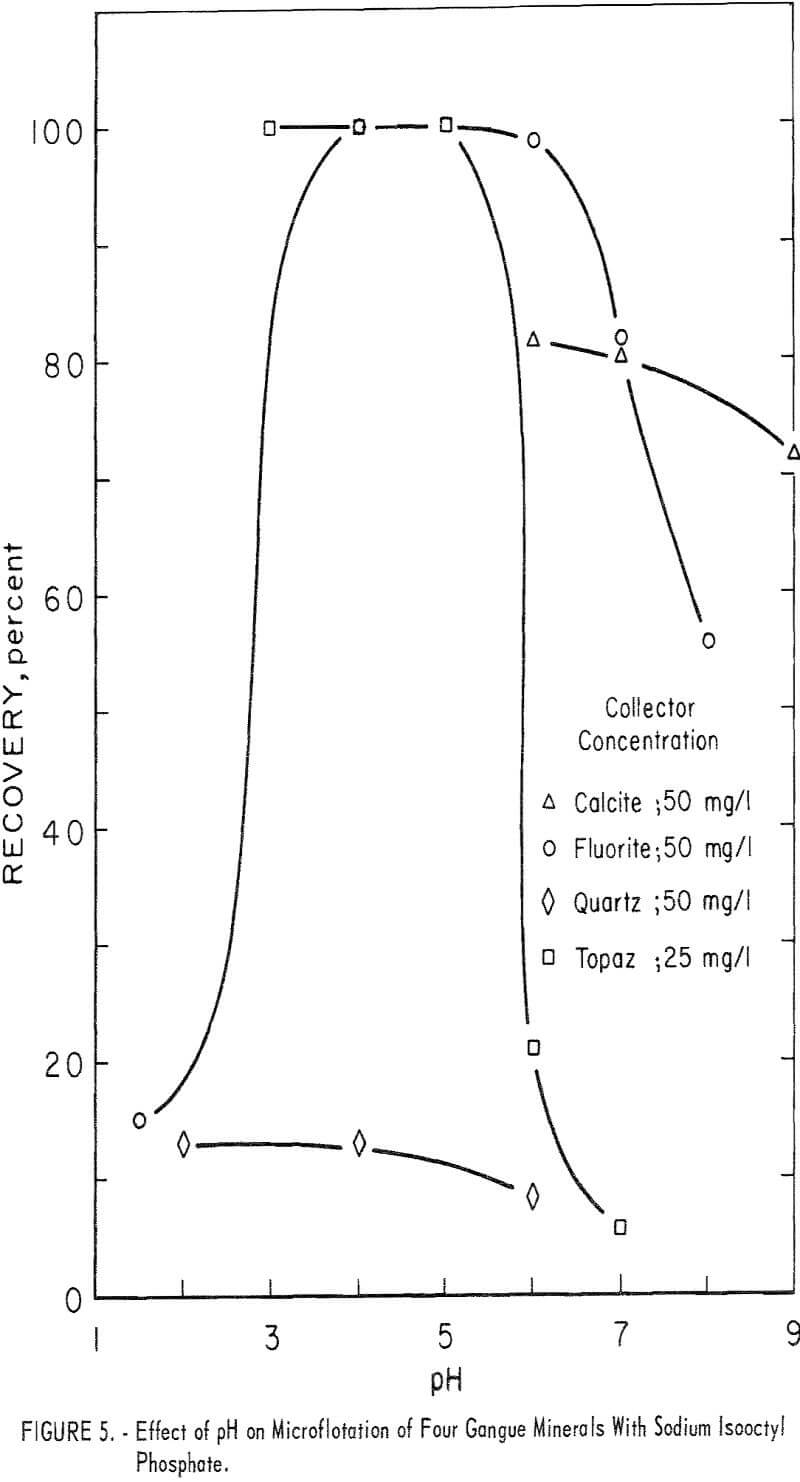
The high recovery ranges of two gangue minerals, fluorite and topaz, overlapped most of the pH range of high recovery for the two beryllium minerals. Data for 5-minute flotation of the gangue minerals calcite, fluorite, and quartz with 50 mg collector per liter and topaz with 25 mg collector per liter are shown in figure 5. Additional data, which shed some light on the flotation rates of the minerals studied, were obtained by observing the mineral recoveries after 1 minute of flotation. These data for 1-minute flotation are presented in table 4, and show a differential in the flotation rate of the several minerals. Feldspar and quartz flotation rate is much lower than that of beryl and chrysoberyl. At pH 4 flotation rate of chrysoberyl and topaz was highest of all minerals tested. Recoveries of fluorite at pH 1.5 for 1- and 5-minute flotation times were 1 and 15 percent, respectively.
With a collector concentration of 100 mg per liter, calcite recoveries ranged from 98 to 100 percent through the pH range 5 to 8. Calcite recoveries at pH 9 and 11 were 91 and 46 percent, respectively. Recoveries of feldspar at the 50 mg per liter were relatively low-38 percent at pH 4.0 and 12 percent at pH 6.0.
Reported in table 5 are the results of the microflotation of beryl, chrysoberyl, fluorite, feldspar, quartz, and topaz with two modifiers. Use of each of these modifiers, Al2(SO4)2 and MgSiF6, depressed beryl, chrysoberyl, and topaz.
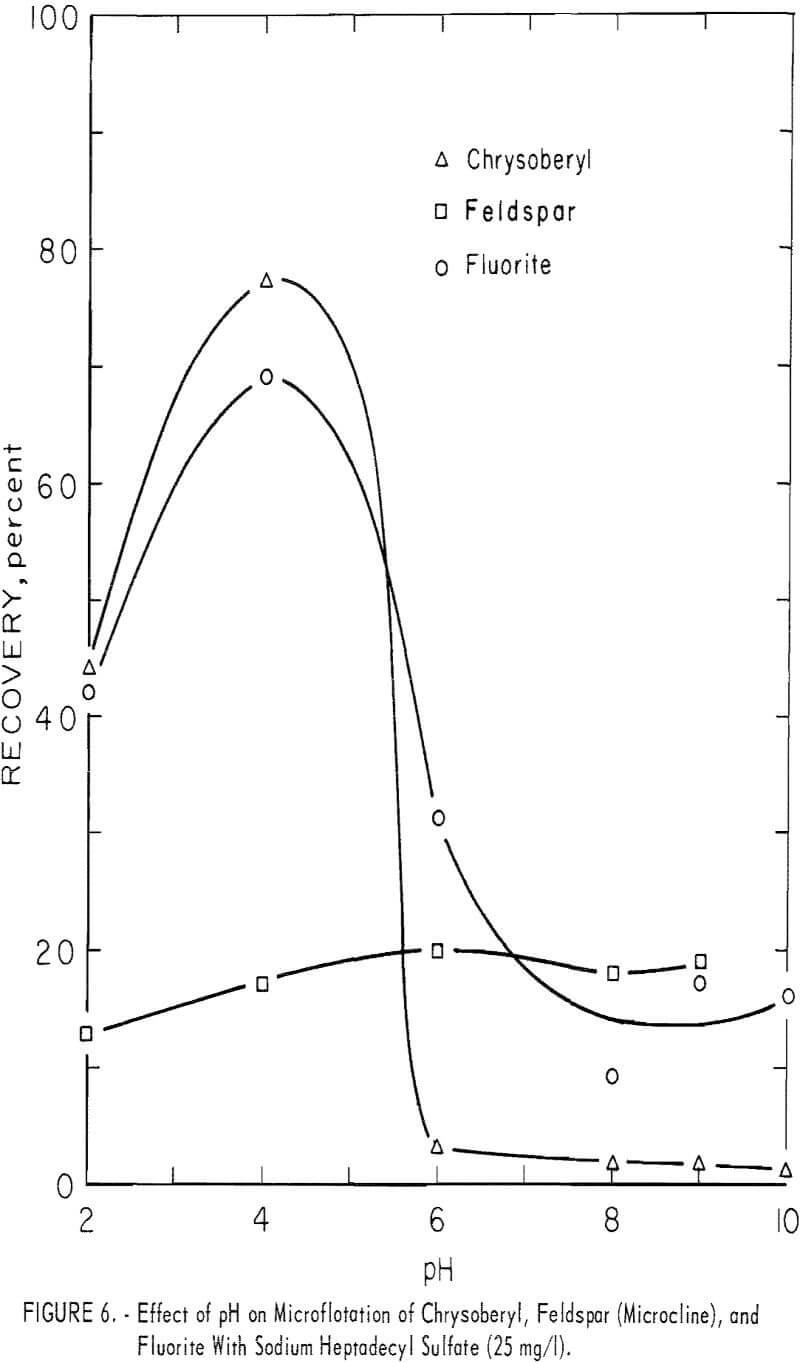
Sodium Heptadecyl Sulfate
The study with sodium heptadecyl sulfate was limited to tests at the 25 mg per liter concentration within the pH range 2 to 10. No tests were made using modifiers. Test solutions were buffered at pH 6 and 8 with 100 mg per liter NaHCO3. Chrysoberyl recovery was highest at pH 4, that is, 77 percent. Chrysoberyl, fluorite, and feldspar results are shown in figure 6. Beryl recovery was 100 percent at pH 2, but dropped to 11 and 2 percent, at pH levels 6 and 8, respectively. Recovery of topaz was complete at pH 2 and 4, but at pH 8 it decreased to 4 percent. Calcite recoveries were 80 and 69 percent, respectively, at pH 6 and 9.
Sodium Alkyl Sulfonate
Studies of sulfonate flotation were limited to chrysoberyl flotation with sodium benzene sulfonate in the pH range 2 to 7. Recoveries were complete between pH 2 and pH 4.5 with 90 mg sodium benzene sulfonate per liter; however, recoveries dropped to 6 percent at pH 6. These microflotation data show better flotation than would be predicted from contact-angle data at the same concentration of reagent.
Sodium Dioctyl Sulfosuccinate
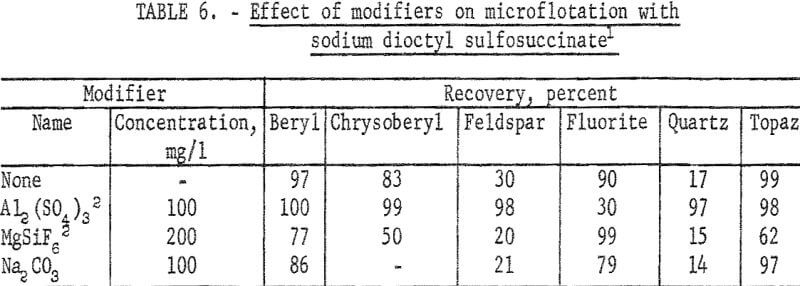
The sodium dioctyl sulfosuccinate study was devoted principally to a test series on beryl, chrysoberyl, and gangue minerals at pH 4 with 30 mg per liter collector and with modifiers. These results are reported in table 6. Additional information was obtained with 60 mg per liter collector and recoveries of both chrysoberyl and topaz were complete at pH 2 and 4. At pH 6 chrysoberyl recovery was 4 percent and topaz recovery 32 percent. Also calcite recoveries were complete at pH 6 and 8.
Cationic Surfactants
Primary Amines
One alpha-amine and one beta-amine were tested for their effectiveness as flotation collectors for beryl, chrysoberyl, and gangue minerals.
Alpha-Amine– The C12 alpha-amine (1-aminododecane) was an effective collector for all minerals at pH 11. It was used without modifiers as the chloride salt in a microflotation series on chrysoberyl, but in a series with modifiers all minerals were tested with the acetate salts of the amine. In all tests the solution was buffered with 100 mg per liter NaHCO3 to maintain the selected pH.
Chrysoberyl recoveries with 2 mg per liter C12 alpha-amine chloride ranged from 6 percent at pH 2 to 73 percent at pH 11. Through the same pH range with 4 mg per liter chrysoberyl recoveries were 31 to 100 percent. Recovery was complete at pH 10 to 11.
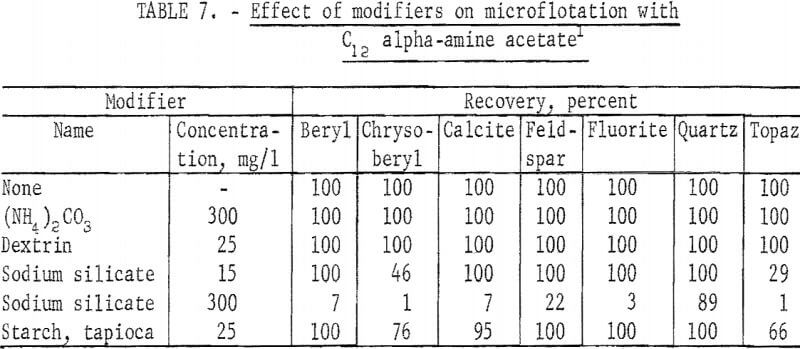
In tests with modifying reagents C12 alpha-amine acetate was used at pH 11 and 4 mg per liter concentration. These results are reported in table 7. Of the five modifiers tested, two -(NH4 )2CO3 and dextrin had no effect on floatability. Chrysoberyl and topaz flotation was strongly depressed by sodium silicate at a concentration of 15 mg per liter. At 300 mg per liter all seven minerals were depressed. This result is apparently due mostly to reduction of effective collector concentration by its reaction with the modifier rather than to specific depressing action on the minerals themselves. Depression by starch at 25 mg per liter was less; again chrysoberyl and topaz were the two minerals showing significant depression.
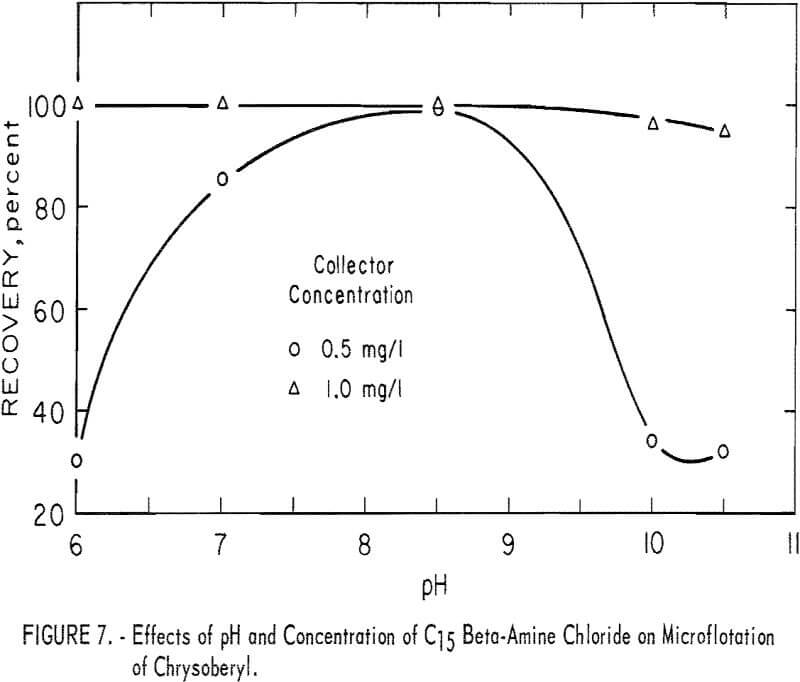
Beta-Amine.– High recoveries of chrysoberyl were obtained at low concentrations of C15 beta-amine (2-aminohendecane). In all tests this amine collector was used as the chloride salt with 100 mg per liter NaHCO3 for pH buffering. Results of microflotation of chrysoberyl at collector levels of 0.5 and 1.0 mg per liter are shown in figure 7. These data show that the most favorable pH range is near pH 8 and that high recovery is obtained with low collector concentration.
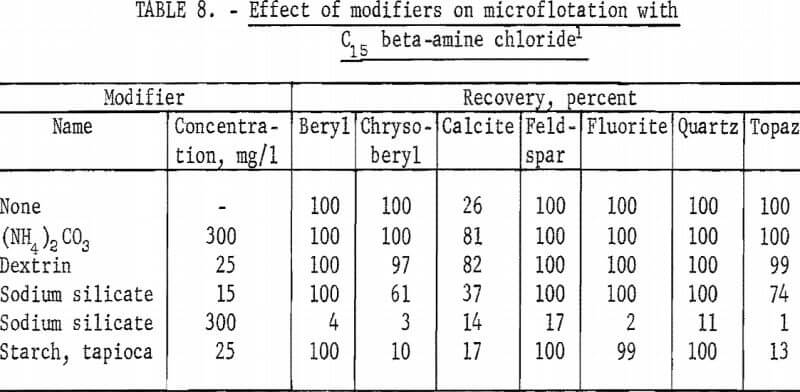
Results of tests with modifiers, reported in table 8, are similar to those obtained with the C12 primary alpha-amine acetate collector. Sodium silicate was the most active depressant, depressing all minerals at 300 mg per liter. With tapioca starch at 25 mg per liter depression of chrysoberyl, calcite, and topaz was significant.
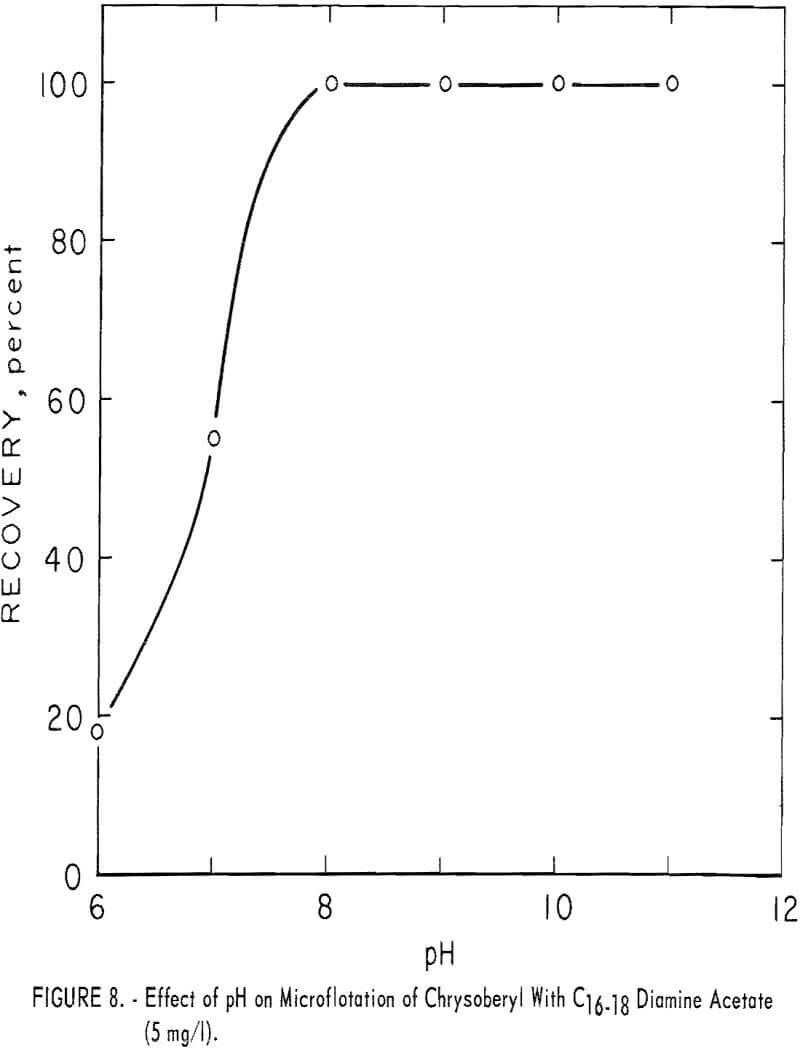
Diamine
The C16-18 diamine acetate, at 5 mg per liter concentration, was an effective collector for chrysoberyl through the pH range 8 to 11. These data are shown in figure 8.
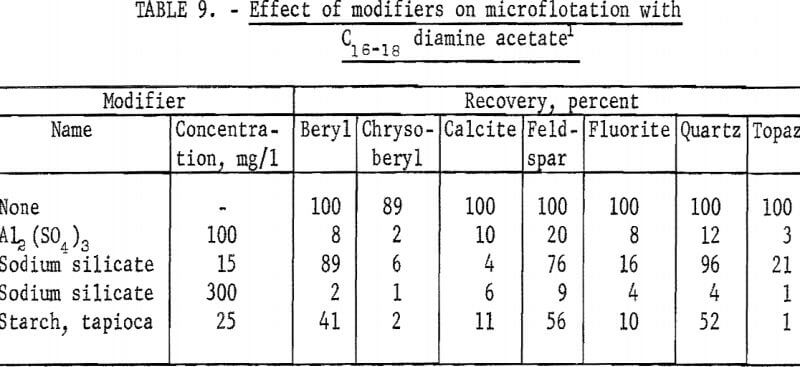
At the same collector concentration of 5 mg per liter the effect of four modifiers on recovery of beryl, chrysoberyl, and five, other minerals was studied. As may be seen in table 9, all modifiers had some depressing effect on each mineral tested.
Tertiary Alkyl Amine
C18-22 tertiary amine acetate was used in a concentration of 10 mg per liter to test microflotation of both beryl and chrysoberyl. At the pH 8 test level the test solutions were buffered with 100 mg NaHCO3 per liter. Beryl recoveries were 100 percent through the pH range 4 to 10. At pH 2 and 11 beryl recoveries were 10 and 89 percent, respectively. Chrysoberyl recoveries were lower; at pH 4, 8, 9, 10, and 11 recoveries were 56, 93, 97, 84, and 82 percent, respectively. Fluorite recovery was essentially 100 percent through pH 2 to 8. Rate of fluorite flotation was rapid at pH 2-95 percent in 15 seconds.
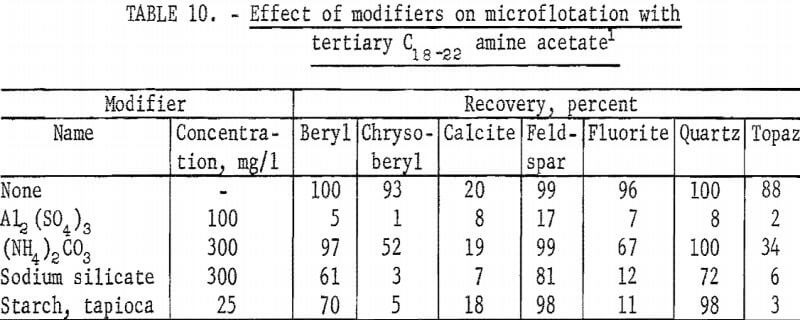
Microflotation tests with modifying agents were made at pH 8 at a collector concentration of 10 mg per liter. Test solutions were buffered with 100 mg NaHCO3 per liter. Aluminum sulfate was the most effective depressant tested. Starch strongly depressed topaz and chrysoberyl flotation but had little effect on feldspar and quartz flotation. Test results with these modifiers are reported in table 10 with those of (NH4)2CO3 and sodium silicate.
Quaternary Amine
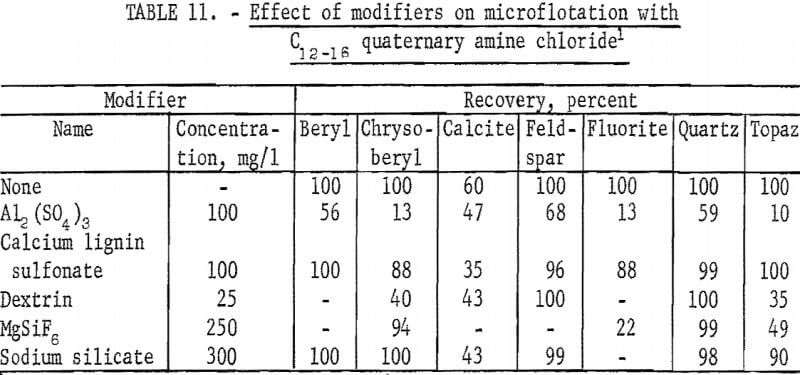
The high recoveries of most of the ore and gangue minerals obtained in tests with the quaternary amine reagent, C12-16 quaternary amine chloride indicate low selectivity for these minerals. The collector was used at a concentration of 12.5 mg of effective amine per liter, and sodium bicarbonate at 100 mg per liter concentration was used to buffer test solutions at pH 6 and 8. Chrysoberyl recoveries were highest between pH 9 and 10 but were generally lower than recoveries of the other minerals, except calcite.
Based on the recoveries for 1-minute flotation time, flotation rate for chrysoberyl was most rapid at pH 10-approximately 70 percent- and for fluorite at pH 9—approximately 45 percent.
A study of the influence of modifiers was made at pH 9 with 12.5 mg Cl2-16 quaternary amine chloride per liter. In control tests, for this series, recoveries for all minerals except calcite were 100 percent. These results are reported in table 11. These data show Al2(SO4)3 and dextrin had the greatest depressing affect on chrysoberyl recoveries. In other tests at the same collector concentration of 12.5 mg per liter, recoveries of chrysoberyl and quartz were essentially complete when 100 mg per liter Na2S was used; moreover, quartz flotation was enhanced with recovery being complete in 15 seconds.