Table of Contents
This report describes an investigation by the Bureau of Mines of flotation techniques for the recovery of chromite from fine-grained disseminated ores. Reported work on the concentration of chromiferous materials shows that flotation has been applied almost exclusively to slime-free pulps. Therefore, chromium recoveries that could be obtained by flotation were related to the amount of slime rejected. However, results of recent studies by the Bureau of Mines showed that desliming is not a requisite to selective concentration of chromite. Laboratory-scale tests on seven ore types from the more important chromium-bearing areas of the Pacific Northwest showed flotation to be technically feasible for treatment of all but one of the samples.
Concentrate grades were affected more by the composition of the chromite mineral than by the grade or character of the ore. Test results from three of the samples showed that, without prior desliming of the pulp, 78 to 91 percent of the chromium could be recovered in concentrates of 90 percent or more chromite; two samples responded to similar treatment, resulting in concentrates of more than 80 percent chromite with recoveries of 85 and 89 percent chromium. One sample of mill products, subjected to severe weathering, required removal of the primary slime to obtain a concentrate of 65 percent chromite with only 75 percent chromium recovery.
The U.S. reserve of chromite is predominantly in low-grade materials that require beneficiation to produce usable products. Considerable research has been conducted on beneficiation by gravity, and successful processes have been developed for recovering the chromite from some of these low-grade materials. However, because of the extremely fine-grained nature of many of the chromiferous materials, recovery by gravity methods was often limited. Therefore, the use of flotation as a method of recovering chromite from low-grade materials was investigated.
A flotation scheme, which introduced the use of a fluoride ion with a fatty-acid collector for selective recovery of chromite, was used in the initial studies. This method, developed and patented by Richard Havens, is applicable primarily to particle sizes between 48- and 200-mesh. The ore types most suited for treatment by this scheme are those consisting of sands, residues from gravity plants, and others of an essentially deslimed nature. As with most conventional flotation procedures for chromite, desliming was a requisite to mineral selectivity and recovery.
Since the ores used in this investigation were essentially of the fine-grained disseminated types, conventional methods of flotation resulted in low chromium recoveries. This study was concerned with modification of the scheme to permit: (1) The flotation of the chromite in the presence of slimes, (2) the application of a collector cheaper than oleic acid, and (3) the preparation of collector reagents in a form suitable for treatment of a variety of ores.
Previous testwork in this laboratory had shown that, in some cases, the addition of fuel oil would improve selectivity during flotation of chromite in the presence of gangue slimes. The collection with fatty acids and fuel oil as reported by Taggart and Arbiter showed that fuel oil alone does not collect, and fatty acid alone gives weak, non-selective collection. The use of both reagents will allow a neutral oil film to support the weakly anchored fatty acid.
John Day Chromites
The chromite deposits in the John Day area of Oregon comprise one of the largest reserves of domestic chromite, containing over 200,000 tons of ore at an average grade of 22 percent Cr2O3 (chromic oxide). Considerable exploration and utilization research have been carried out on these deposits. The feasibility of using these ores as a source of ferrochrome and refractories has been studied.
Most of the reserves in the area are in the George ten and Billie Girl deposits, formerly the Chambers and Iron King deposits, respectively. The chromium is present in an impure chromite; in many cases, the low chromium content of the mineral precludes the preparation of high-grade concentrates. The chromite also is fine grained and intimately associated with the gangue constituents.
Chromite samples were received from three deposits in the John Day area of Grant County, Oreg. In addition to the George Len (Chambers) and Billie Girl (Iron King) samples, a third sample was obtained from the Big Jim (Marks and Thompson) deposit.
Nature of Ores
Physical
In the George Len claim, the chromite occurs in a dark-brown, locally yellow-weathering, fine-grained dunite. In some places, the host rock ranges from dunite to aporphyritic peridotite, and in others it becomes more or less completely serpentinized. Petrographic examination showed the test sample to consist essentially of chromite, with some associated ferromagnesian silicate minerals (primarily clinopyroxene) and chlorite (ripidolite), and a relatively small amount of antigorite. Maximum liberation of the chromite occurs in the minus 100- plus 200-mesh fraction, where there is evidence of only minor locking with the gangue minerals.
The chromite in the Billie Girl claim is distributed through a dark-green, completely serpentinized host rock, probably an altered peridotite. There are many shears, faults and breaks in the chromiferous material, and in places it is hard to distinguish the material from the dark serpentine. Petrographic examination of the test sample showed it to consist primarily of chromite, with some chlorite and a small amount of serpentine. Also present were traces of olivine, magnetite, pyroxene, and ankerite. An examination of the screened fractions indicated that locking of the chromite is present in all fractions, including the minus 400-mesh. Microscopic grain counts, however, revealed that approximately 70 percent of the chromite is liberated in the minus 325- plus 400-mesh fraction.
The Big Jim chromite occurs disseminated in a matrix of serpentinized peridotite, with local segregations of massive chromite. Petrographic examination of the test sample revealed the presence of chromite and serpentine, with small amounts of associated olivine, pyroxene, chlorite, and dolomite. A study of sized fractions indicated that chromite locking is prevalent in all sizes, including the minus 200-mesh. However, about 80 percent of the chromite is liberated in the minus 100- plus 200-mesh fraction.
Chemical
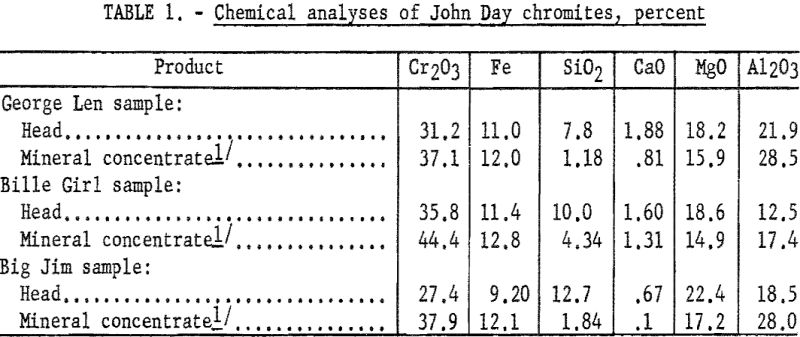
The chemical analyses of head samples of the ores tested are given in table 1. Analyses of high-purity mineral concentrates are also included to show the impure nature of the chromite mineral. Spectrographs analyses of the head samples are given in table 2.
Flotation
Since the impurity of the chromite contained in these samples precluded concentration to present commercial specifications by physical methods, the test objective was to determine chromium recovery in products of plus 90 percent chromite. Highly concentrated chromite fractions were produced from each of the three samples. Chromium recovery was about 90 percent from the George Len and Billie Girl samples; however, only 77 percent of the chromium was recovered from the Big Jim sample. Although the concentrates obtained from the first two samples contained 70 to 80 percent of the feed weight, entrainment of fine gangue in the oiled flocs was not sufficient to prevent attaining products in excess of 90 percent chromite. The selectivity of the scheme is comparable to that obtained in usual sulfide flotation practice.
George Len Ore
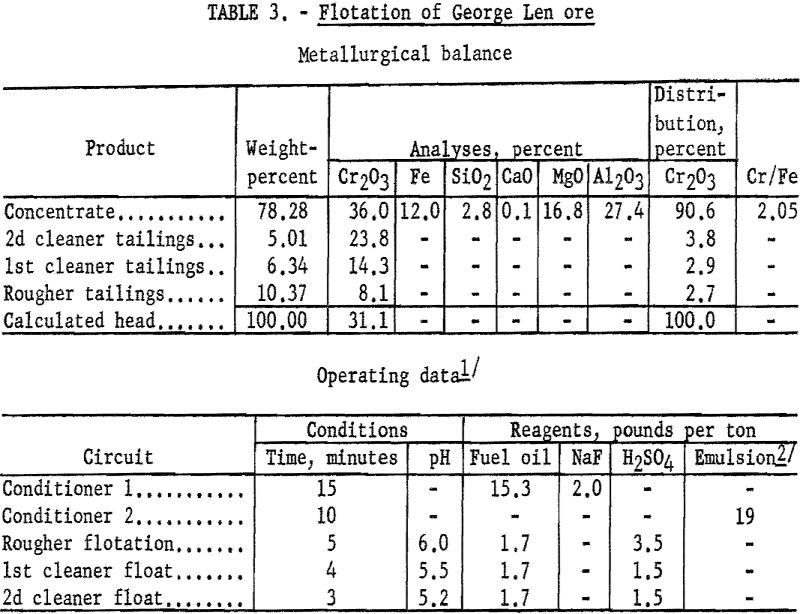
A series of tests on the George Len sample demonstrated that 90 to 93 percent of the chromium could be recovered by flotation in concentrates containing from 34.6 to 36.2 percent Cr2O3. A typical test procedure and its results are described.
One-kilogram samples of George Len ore were stage-ground to 97 percent minus 100-mesh. The ground pulp was preconditioned with 2 pounds of sodium fluoride and 15.3 pounds of fuel oil per ton of ore for 15 minutes. Conditioning was then extended 10 minutes after the addition of 19 pounds of tall oil-petroleum sulfonate emulsion per ton of ore. The flocculated chromite minerals were floated in a rougher stage in which 3 pounds of sulfuric acid per ton of ore was added at the start. Reagent additions of fuel oil and sulfuric acid were 1.7 and 0.5 pounds, respectively, per ton of ore during rougher flotation. The concentrate contained 90.6 percent of the chromium at a grade of 36.0 percent Cr2O3. Based on a 99-percent pure-mineral assay of 37.1 percent Cr2O3, the mineral content of the concentrate was calculated at 96 percent chromite. Tables 3 gives complete metallurgical data,
Billie Girl Ore
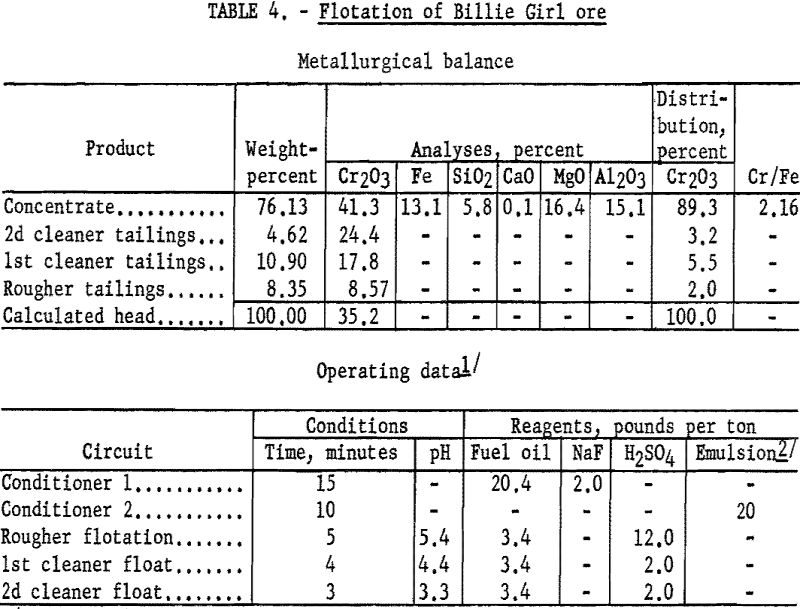
Flotation concentrates from the Billie Girl sample contained 83 to 90 percent of the chromium at grades of 39.5 to 42.4 percent Cr2O3. Concentrate grades as high as 44.9 percent Cr2O3 were obtained, but the recoveries decreased sharply to approximately 50 percent. The test procedure and results of a typical test are described below.
Flotation procedure on the Billie Girl sample was the same as for the George Len sample, except that a larger amount of slimes present made more reagent necessary, and no sulfuric acid was added during the rougher flotation step. In the conditioning steps, 20.4 pounds of fuel oil and 20 pounds of tall oil-petroleum sulfonate emulsion per ton of ore were used. The concentrate contained 89.3 percent of the chromium at a grade of 41.3 percent Cr2O3. By calculation, the concentrate contained 90 percent chromite mineral. Complete metallurgical data are given in table 4.
The Billie Girl sample also responded to the flotation technique described in the following section for the Big Jim sample. Results were comparable to those given in table 4.
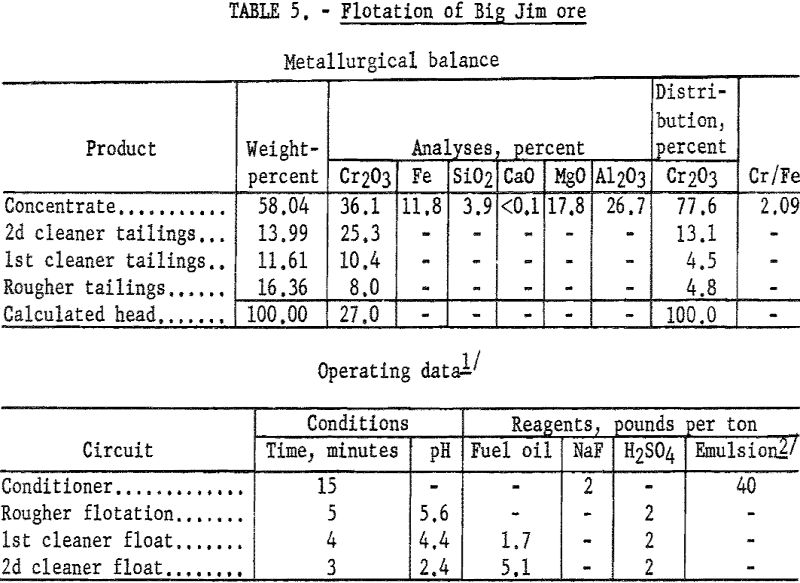
Big Jim Ore
Preliminary testwork on the Big Jim sample showed that mineral selectivity was greatly reduced when using the scheme successful on the other two John Day ores. When enough fuel oil was added to flocculate the gangue slimes, the pulp became so heavily oiled that no separation of minerals was made. Incorporating the fuel oil into the collector emulsion reduced the total amount of fuel oil required and permitted the selective flotation of the chromite.
Concentrates produced by flotation contained 34.7 to 36.8 percent Cr2O3 at chromium recoveries of 65 to 79 percent. The small sample available at the time of the investigation prevented more detailed study to improve chromium recoveries in concentrates containing 90 percent chromite. The test described is one which involves the simplest flotation procedure that resulted in the desired concentrate purity and represents the highest recovery obtained by this scheme.
One-kilogram samples of ore were stage-ground to pass 100-mesh. The pulp was conditioned for 15 minutes with 2 pounds of sodium fluoride and 40 pounds of emulsified collector oils per ton of ore. The pulp was made acidic by the addition of sulfuric acid. The flocculated chromite minerals were floated, and the rougher concentrate was cleaned twice. The final concentrate contained 77.6 percent of the chromium at a grade of 36.1 percent Cr2O3. By calculation, the concentrate contained approximately 90 percent chromite. Table 5 gives complete metallurgical data.
Twin Sisters Chromites
In the Twin Sisters area, dunite outcrops over an area of approximately 45 square miles, Chromite is found in lenses and scattered along zones or bands of varying width. Selected grains of chromite assay as high as 51 percent Cr2O3.
Two samples of ore were received from the Twin Sisters deposit. The samples represented some of the very low-grade ore (Alamether claim) and some of the best grade (Gora claim).
Nature of Ores
Physical
The Gora sample consisted primarily of chromite and olivine, changing to serpentine with a small amount of orthopyroxene. The olivine ranged in color from green to yellowish-brown.
The chromite is liberated essentially in the minus 100-mesh fraction; however, a small amount of chromite remains locked in the minus 325-mesh fraction.
The Alamether sample was a dunite consisting primarily of olivine, with a small amount of disseminated chromite. Very small amounts of orthopyroxene and chlorite also were observed.
A study of sized fractions indicated that the chromite is almost entirely liberated in the minus 48- plus 65-mesh fraction.
Chemical
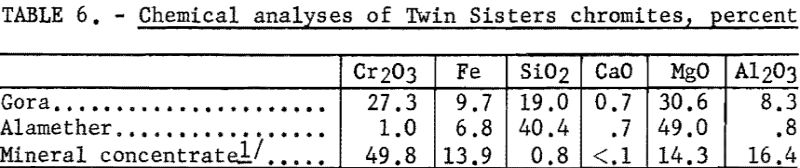
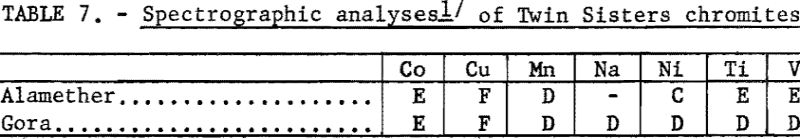
The chemical analyses of the head samples of the Twin Sisters deposit are given in table 6. The analyses of a high-purity mineral concentrate from the Gora claim ore are also given. Spectrographs analyses of the head samples are given in table 7.
Flotation Gora Ore
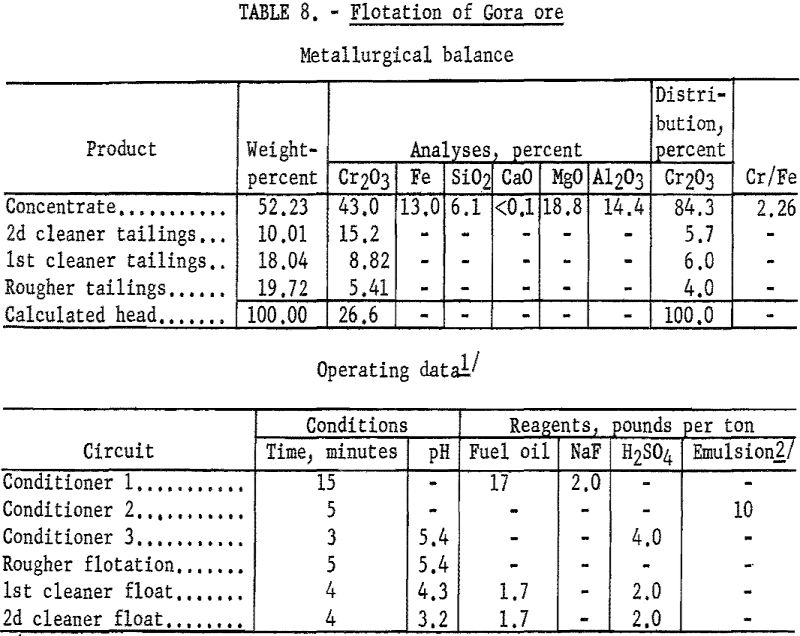
Chemical analysis of the chromite mineral prompted investigation of using flotation to produce a concentrate containing 44 to 48 percent Cr2O3. The best results obtained in this phase of the study showed a concentrate grade of 45.6 percent Cr2O3 at a chromium recovery of 75.8 percent. Lowering the concentrate grade to 42 percent Cr2O3 increased recoveries to approximately 85 percent. The test procedure and results described are representative of the flotation characteristics of the Gora ore.
One-kilogram samples of Gora Claim ore were stage-ground to minus 100- mesh. The ground pulp was conditioned in three stages: (1) The pulp was in contact for 15 minutes with 2 pounds of sodium fluoride and 17 pounds of fuel oil per ton of ore; (2) the pulp was agitated for 5 minutes with 10 pounds of tall oil-petroleum sulfonate emulsion per ton of ore; and (3) the pulp acidity was adjusted to a pH of 5.1 by conditioning it for 3 minutes with 4 pounds of sulfuric acid per ton of ore. The flocculated chromite was floated for 5 minutes in the rougher circuit. By cleaning the rough concentrate twice with additions of fuel oil and sulfuric acid, 84.3 percent of the chromium was recovered at a grade of 43.0 percent Cr2O3. Complete metallurgical data are given in table 8.
Alamether Ore
Because of the low tenor of the Alamether sample, only preliminary tests were conducted. Metallurgical results were not encouraging.
Mouat Chromites
The Mouat chromite deposit is situated in an area geologically known as the Stillwater Complex, along the north flank of the Beartooth Mountain Range in south-central Montana. This complex contains the largest known chromite deposits in the United States.
The American Chrome Co. began production of chromite concentrates in August 1953. Approximately 1,000 tons of ore are mined per day from two ore bodies designated as the G and H veins. The ore bodies occur as lenses in poikilitic harzburgite and grade from massive to disseminated chromite from the foot wall to the hanging wall, respectively. The millfeed averages about 20 percent Cr2O3. Hydraulic classification and tabling of ore ground to minus 20-mesh is used to concentrate the chromite. Reported mill results show an average concentrate grade of 38.5 percent Cr2O3 at a recovery of 86 percent. The chromite mineral, with an average analysis of less than 45 percent Cr2O3, limits the grade of concentrate to about 38.5 percent Cr2O3.
Two samples of material were received from the Mouat deposit; one represented the feed to the mill and the other the overflow from the hydraulic classifiers. The overflow is used as the feed material for the fine-table section.
Nature of Ore
Physical
The Mouat ore samples consist essentially of chromite and olivine, with small amounts of associated amphibole, serpentine, magnesium-replaced calcite, and chlorite. Very small amounts of pyroxene, feldspar, and magnetite also are present.
Because of the apparent variability in size of the chromite contained in the raw ore, there is much locked and liberated chromite in all sized fractions of minus 10-mesh ore.
The fine table feed as received was about 98 percent minus 100-mesh. A size analysis of the material is given in table 9.
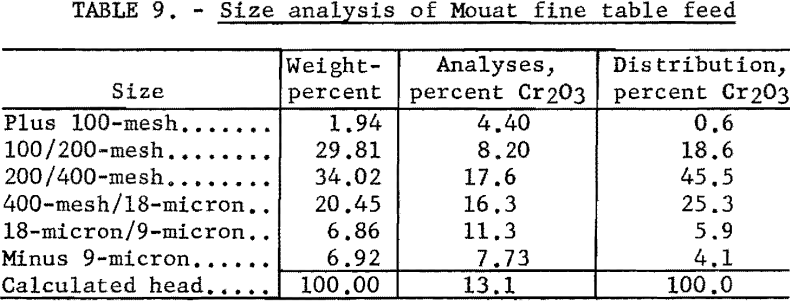
The sample of fine table feed consisted essentially of olivine, less biotite, plagioclase, pyroxene, dolomite, and chromite, and relatively small to very small amounts of chlorite and serpentine.
Chemical
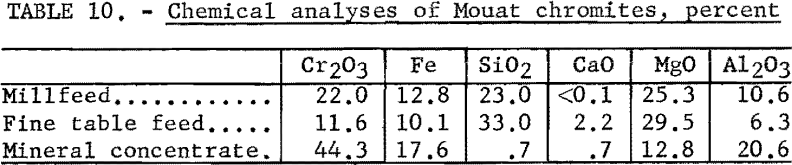
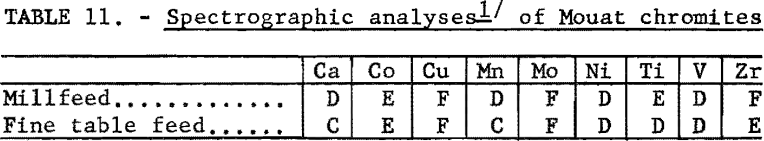
The chemical analyses of the millfeed and fine table feed are given in table 10. The average analysis of the chromite mineral found in the Mouat ore is also given. Table 11 gives the spectrographs analyses of the two samples tested.
Flotation Millfeed
Flotation studies were directed toward obtaining a concentrate grade of approximately 38 percent Cr2O3. However, flotation concentrates consistently graded from 35.0 to 36.9 percent Cr2O3. The desired grade of concentrate was not obtained even at a great sacrifice in recovery. Testwork showed that chromite particles larger than 65-mesh did not respond satisfactorily to flotation. Although the chromite is liberated essentially when the ore is ground to 20-mesh, very poor selectivity resulted in flotation of 48-mesh material. The test procedure and results described are representative of flotation studies.
Representative 1-kilogram samples of Mouat millfeed were stage-ground to minus 65-mesh. The ground pulp was conditioned for 5 minutes with 4 pounds of sodium fluoride and 8 pounds of sulfuric acid per ton of ore. The pulp was then conditioned for 30 minutes with 35 pounds of emulsified fuel oil-tall oil-petroleum sulfonate per ton of ore. Flotation of the flocculated minerals was rapid. One cleaning of the rougher concentrate resulted in a product which contained 88.9 percent of the chromite at a grade of 36.2 percent Cr2O3. Complete results are presented in table 12.
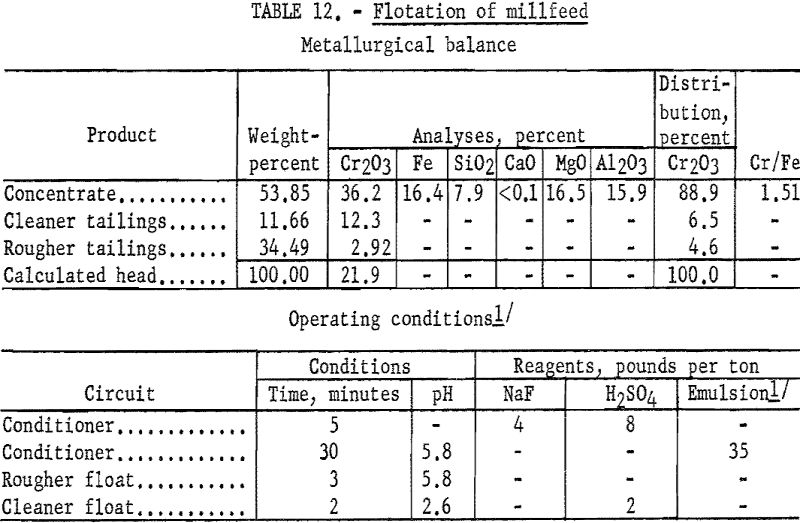
The Mouat millfeed, when ground to 65-mesh, responded to the procedure described for the George Len, Billie Girl, and Gora ores to give about the same results as those in table 12.
Fine Table Feed
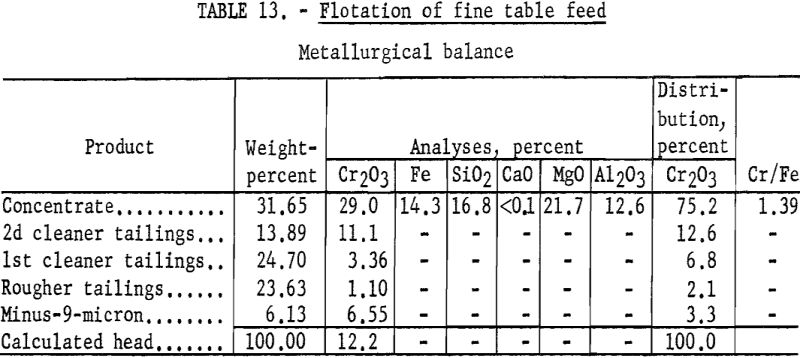
The feed to the fine table section of the American Chrome Co. mill is reported to comprise about 25 percent of the original millfeed. This section produces a finished concentrate lower in grade than the total concentrate. Tailings from the fine table section account for more than 50 percent of the total chromium lost in milling. The samples of the fine table feed, about 98 percent minus 100-mesh and 34 percent minus 400-mesh, were dried prior to shipment to Albany. This sample did not respond to any of the flotation techniques used on the John Day and Twin Sisters chromite samples. However, testwork demonstrated that removal of the minus 9-micron (theoretical 1600-mesh) material permitted flotation of the chromite. Sizing tests showed that an average of 4.1 percent of the chromium was contained in the minus-9-micron material.
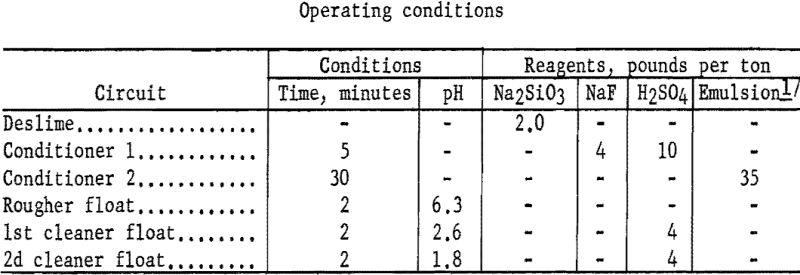
Representative 1-kilogram samples were scrubbed in a laboratory flotation cell at 40 percent solids for 5 minutes to break up the compacted lumps. Sodium silicate was added to the scrubber to disperse the fine material. The scrubbed pulp was sized by sedimentation to remove the minus 9-micron material. The sands were then conditioned for 5 minutes with 4 pounds of sodium fluoride and 10 pounds of sulfuric acid per ton of ore. Conditioning was extended for 30 minutes with the addition of 35 pounds of emulsified fuel oil-tall oil-petroleum sulfonate per ton of ore. The rougher concentrate was cleaned twice with the addition of sulfuric acid, and the final concentrate contained 75.2 percent of the chromium at a grade of 29.0 percent Cr2O3. Complete results are given in table 13.
To determine effects of alteration resulting from weathering and drying, flotation studies were made on raw ore prepared in the laboratory to simulate mill practice. The procedure consisted of stage-grinding samples of millfeed to minus 20-mesh and separating the minus 100-mesh material for flotation testing. The simulated fine table feed contained an average of 16.5 percent Cr2O3 compared with 11.6 percent Cr2O3 for the mill product. Employing the flotation technique described for the George Len, Billie Girl, and Gora ores resulted in concentrates that contained 75 to 84 percent of the chromium at grades of 24.2 to 31.9 percent Cr2O3.
Conclusions
Flotation can be used to recover chromite from many fine-grained disseminated ore types without prior desliming of the ground pulp. Of the seven samples tested, all but the altered feed to the Mouat fine table section responded to flotation for selective recovery of chromite. Removal of the minus 9-micron fines from the fine table feed was necessary to obtain mineral collection and separation. In some ores, the addition of fuel oil and a fluoride ion in a preconditioning step will selectively flocculate gangue slimes and permit their subsequent depression with acid. Concentrate grades were affected more by the purity of the chromium mineral than by the chromium content of the ores. Mineral selectivity by this scheme is comparable to that obtained with anionic collection of sulfide minerals.
