Because of higher efficiency, wet grinding is more widely used than dry grinding, but leads to an increased consumption of grinding media due to corrosion in some ores. In dry grinding, the wear rate of high chromium cast irons is frequently ten times lower than cast or forged steel with a cost ratio of 2-3 times; economic justification is not difficult and explains the dominance of high chromium irons.
A series of Cr-bearing cast iron balls, having a range of Cr content from 0-29% (0%, 8.5%, 11%, 16%, 26% and 29% Cr) was used in this investigation in order to cover a wide spectrum of microstructures and media hardness, resulting in a suitable range of corrosion and abrasion resistance that may be helpful in understanding the responsible wear mechanisms in Fe-Cr-C based materials.
Electrochemical measurements
A grinding ball was made into an electrode by attaching a cone and rod to the opposite sides of a sample to permit high speed rotation. The parallel surfaces were needed for the grinding balls to fabricate in this manner. The top and bottom surfaces of the ball were ground flat and parallel using an abrasive wheel. A lucite tube of 6.25 mm inner diameter was attached to the one flat surface for electrical contact, and a PVC cone having diameter and height of each 5 mm was attached to the other surface using 3M Scotch-weld structural adhesive. The electrical connection was then made by connecting to copper wire with a mercury drop.
Laboratory flotation tests
To relate the wear characteristics, electrochemical behaviors and surface characteristics of various Cr- containing cast irons, a series of batch flotation tests was performed on freshly ground artificial mixtures of 95% quartzite and 5% pyrrhotite in a Denver laboratory flotation cell. The mixtures in the amount of 600 grams were ground at 60% solids to minus 100 mesh in a 203 mm (8 inch) diameter porcelain mill containing 126 grinding balls of each type of cast iron, all nominally 25.4 mm (one inch) in diameter under either a nitrogen or oxygen atmosphere. Potassium ethyl xanthate was used as a collector in the range of 0.01 to 0.1 kg/t. The flotation tests were carried out for 3 minutes, using nitrogen for aeration in order to prevent the oxidation of abraded steel debris during flotation.
Results
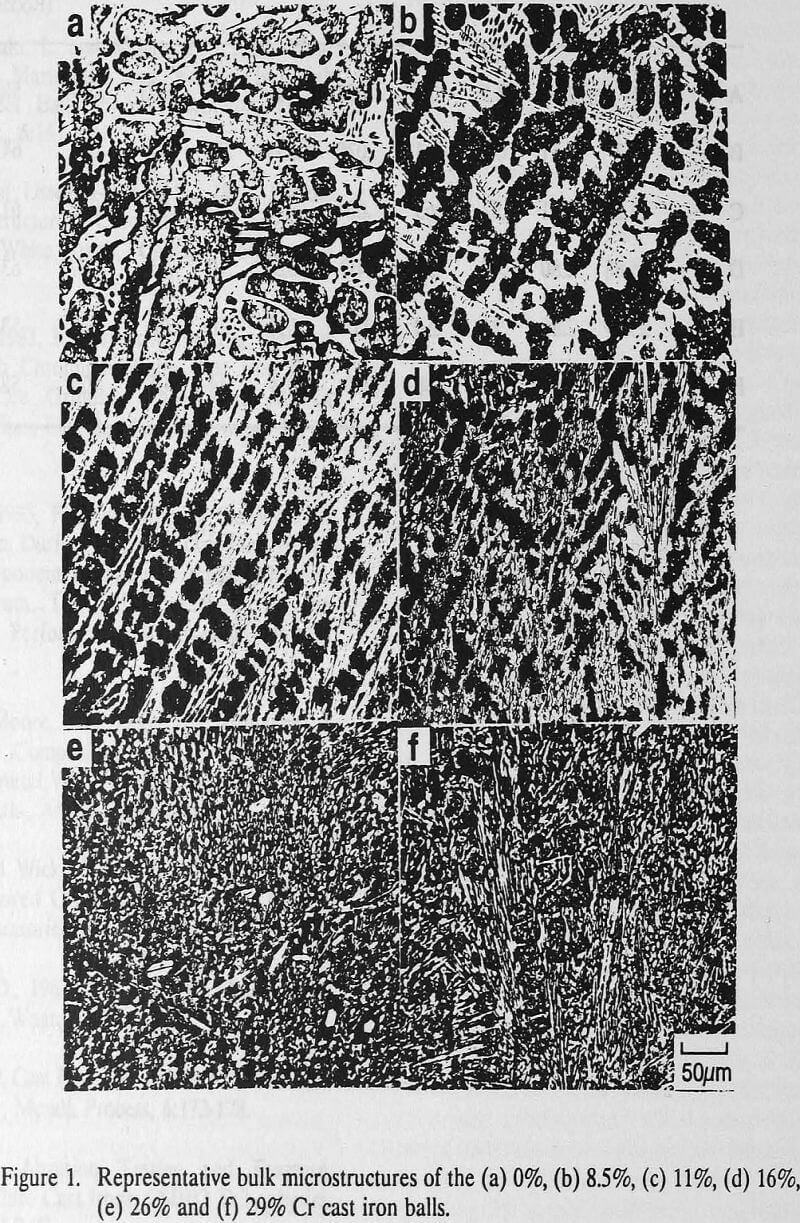
Representative microstructures of the grinding media used for marked ball wear tests show the microstructures of 0% and 8.5% Cr cast irons which consist of dendrites of transformed martensite and retained austenite with interdendritic carbides (FeMn)3C and (FeCr)3C (white), respectively, and ledeburite eutectic comprised of hard brittle iron carbide containing rods and globules of martensite and retained austenite.
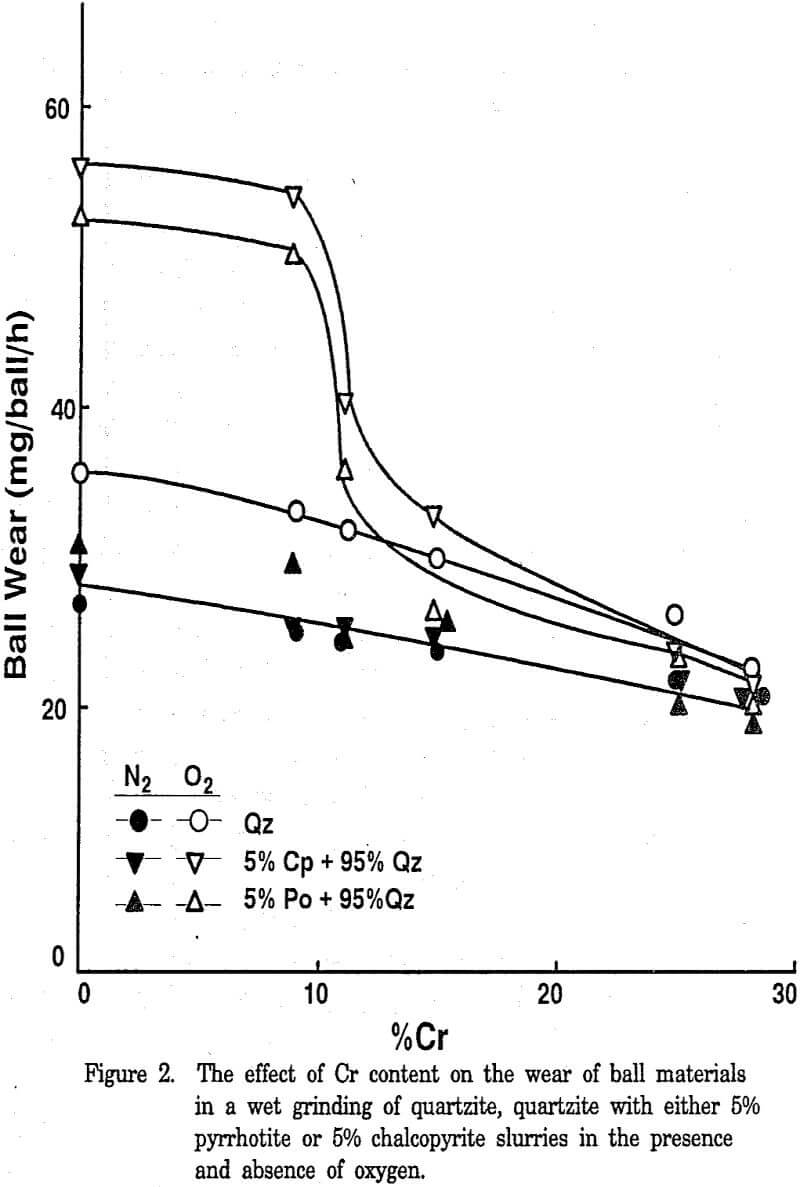
The wear data obtained for the various cast iron balls with the artificial mixtures of quartzite and quartzite with either 5% pyrrhotite or 5% chalcopyrite samples under a nitrogen or oxygen atmosphere. In a nitrogen atmosphere, the ball wear was essentially unaffected by the presence of sulfide minerals. A slight decrease in wear with increasing Cr contents may be related to the abrasion resistance of the ball materials.
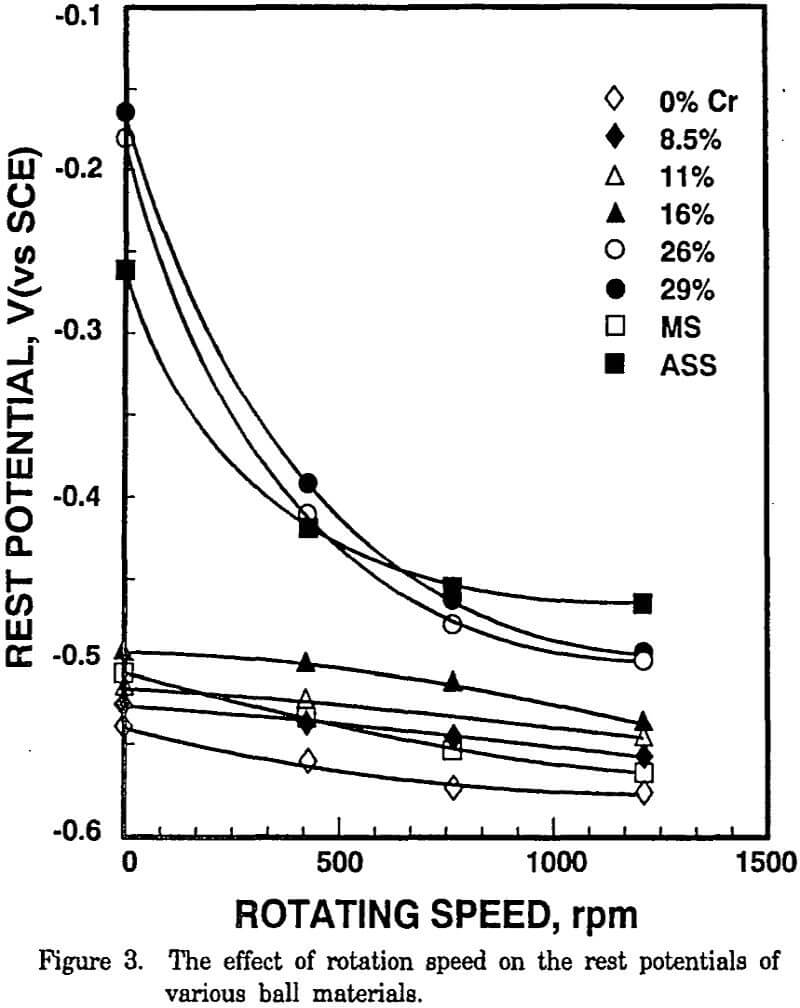
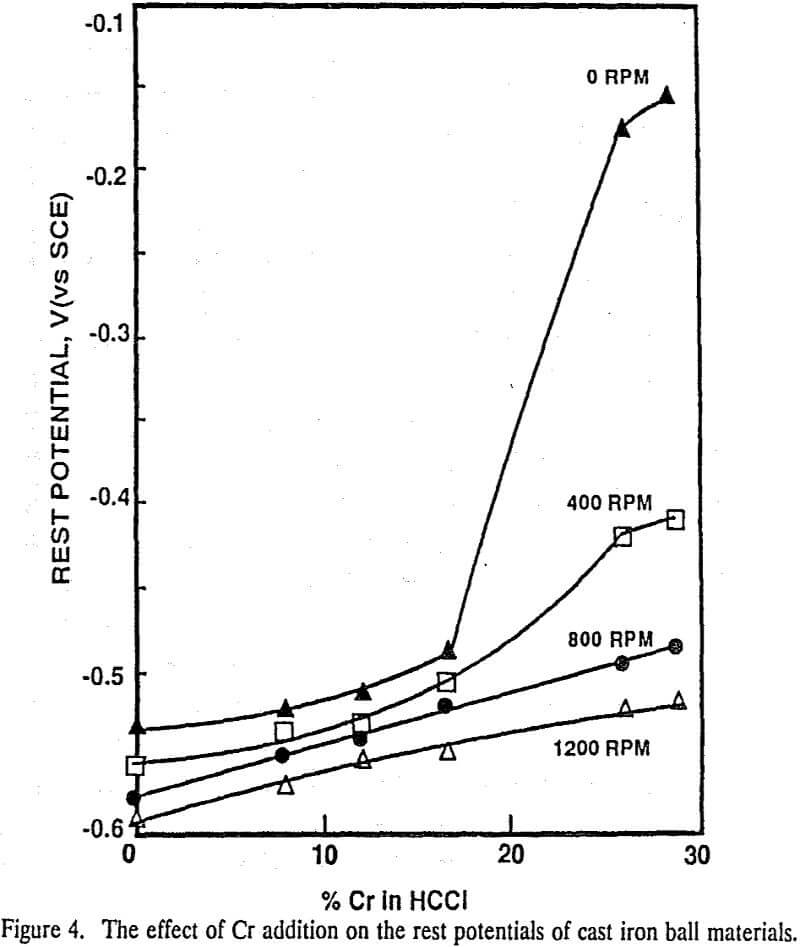
To simulate the abrasive action of a grinding mill on mineral particles and grinding media, a series of electrochemical experiments was carried at various rotation speeds. The rest potentials of high Cr cast iron (26% and 29% Cr) and austenitic stainless steel balls decreased markedly with an increasing rotation speed, whereas very little change in the rest potentials was observed with those of low Cr cast iron (0%, 8.5%, 11% and 16% Cr) and mild steel balls.
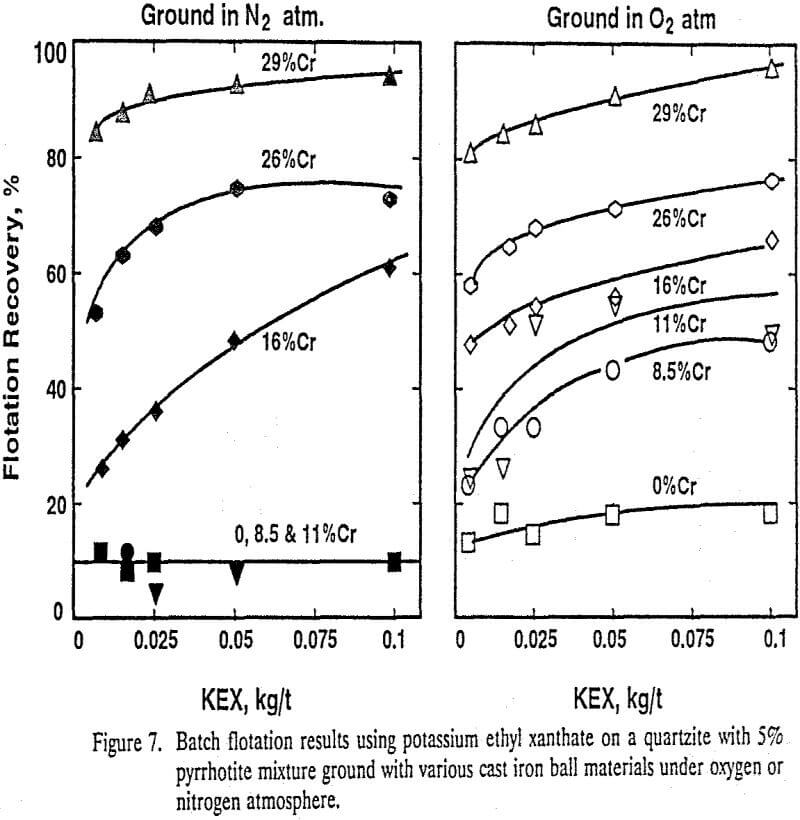
Pyrrhotite ground with the cast iron balls containing less than 11% Cr in a nitrogen atmosphere did not float much even when as high as 0.1 kg/t potassium ethyl xanthate was used, but grinding in an oxygen atmosphere improved the flotation recoveries.
Discussion
The cast iron ball materials have high hardness values because of their high carbon contents. However, the bulk hardness alone will not determine the wear resistance of the material; the chemical composition as well as microstructure are important in determining the wear resistance of a ball material. But the high hardness of cast irons has an advantage over softer grinding media in reducing the wear.
The significance of microstructure to corrosion behavior is that inhomogeneous structures may enhance corrosion due to possible electrolytic cell reaction between second phase particles and matrix, whereas homogeneous structures are relatively free of internal cell action. However, mixed microstructures may wear better or worse than single phase microstructures depending on the relative amount of each phase present. All structures are, however, susceptible to external cell action with ore minerals.
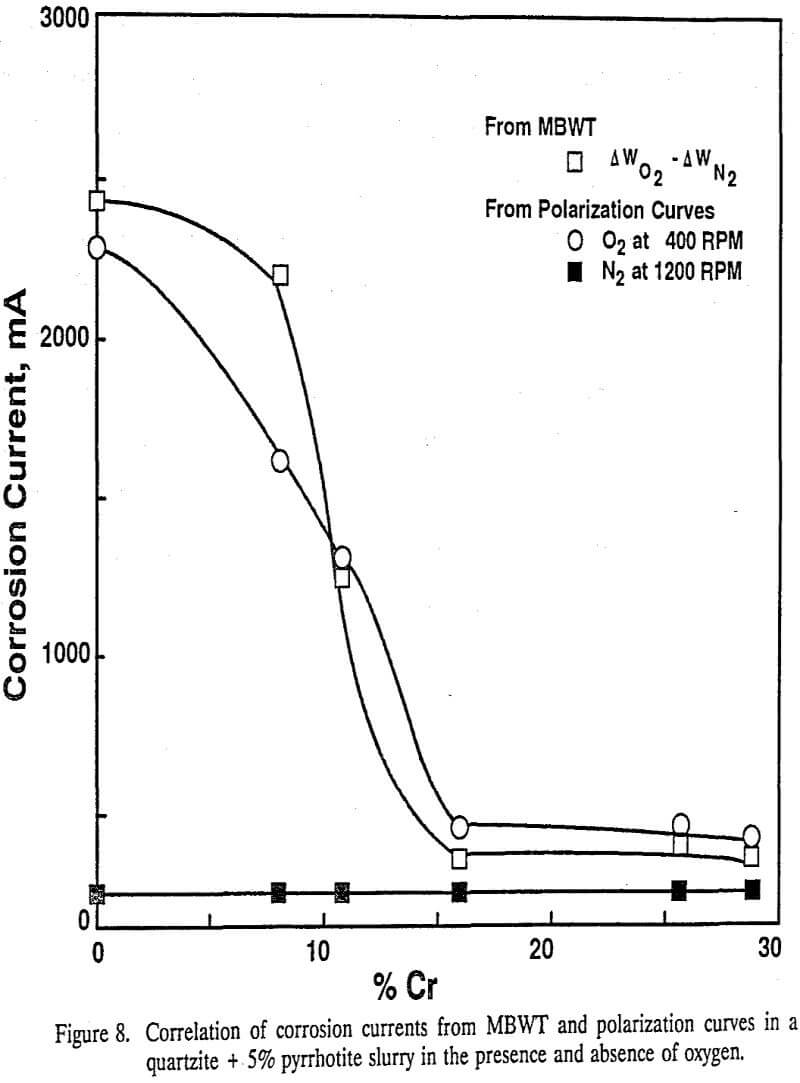
The difference in wear under oxygen and nitrogen atmospheres (assumed to be corrosive wear) increased with decreasing Cr content, which further supports that both the microstructure and the hardness (which in turn depend on the chemical composition and processing conditions) are important in determining the wear resistance of materials. The ball wear increased markedly below 10% Cr in an oxygen atmosphere in the presence of sulfide minerals. Marked ball wear tests indicated that high Cr balls have higher wear resistance than low Cr balls due to the difference in their passivation behaviors.
In wet grinding, nascent metal as well as mineral surfaces are continually being exposed and repassivated due to the removal of passive film by abrasive particles, thereby leading to intense corrosion. These passive films either take some time to rebuild or continually being abraded depending on the type of abrasive, mill speed, chemical composition and hardness of ball materials used during wet grinding.
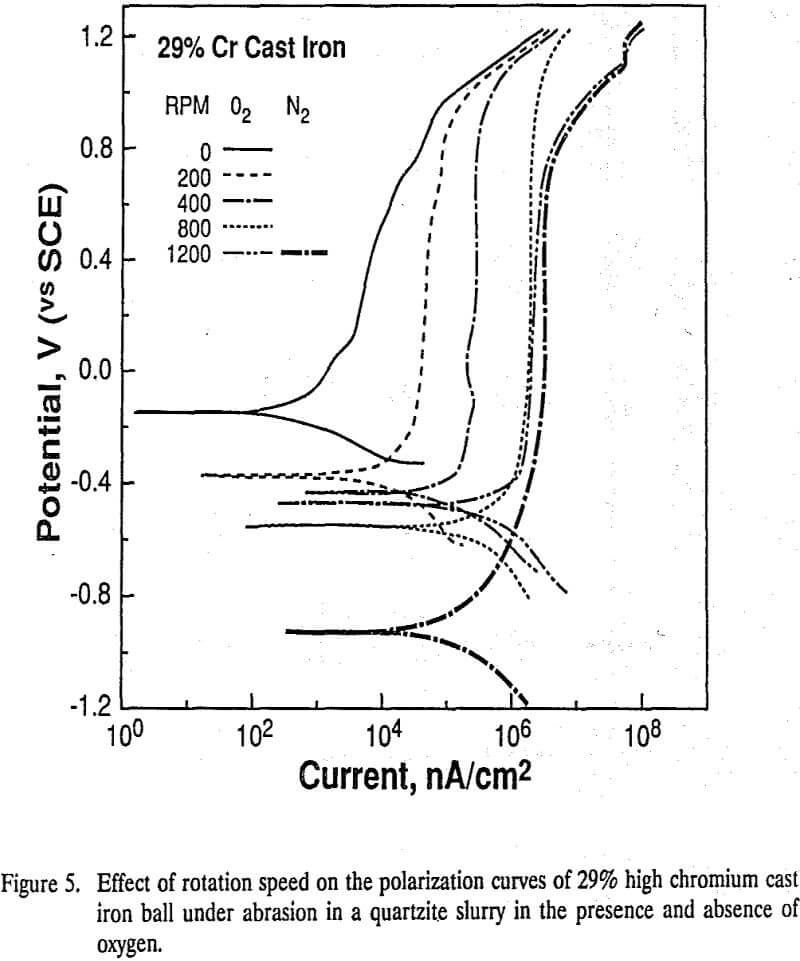
Assuming that balls in the grinding mill rotate at the same peripheral speed as the mill wall, the rotation speed of the balls was estimated to be 400 rpm. Hence, the rotation speed of 400 rpm was chosen to estimate the total corrosion current in oxygen atmosphere from the polarization curves. Under a nitrogen atmosphere, the balls were assumed to be active at all rotation speeds, but the corrosion currents estimated from the polarization curves in a nitrogen atmosphere are seen to be minimal
The high chromium ball (ASS, 26% and 29% Cr) surface was passivated in the absence of abrasion. With increasing rotation speed, the anodic current increased and the rest potentials became more active. With low chromium balls (MS, 0%, 8.5%, 11% and 16% Cr), the anodic polarization curves and rest potentials were affected very little by the rotation speed and the currents.