Table of Contents
- Chlorine Gas Laboratory Experiment I
- Laboratory Chlorine Gas Experiment II
- Laboratory Experiment Chlorine Gas III
- Chlorine Gas Laboratory Experiment IV
- Chlorine Gas Laboratory Experiment V
- Chlorine Gas Laboratory Experiment VI
- Laboratory Experiment with Chlorine Gas VII
- Laboratory Experiment using Chlorine Gas VIII
- Oxygen Derivatives of Chlorine
Chlorine is a heavy gas, being about 2½ times heavier than air, so that it can be collected by downward displacement.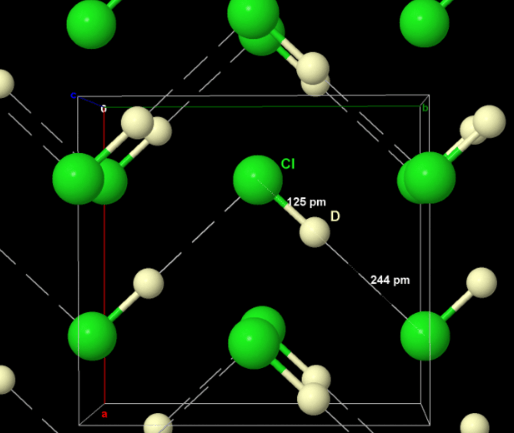
Weigh out about 30 grams of manganese dioxide and about the same quantity of sodium chloride; mix them well together in a mortar, and transfer to a large flat-bottomed flask. Fix up your apparatus as for the preparation of carbon dioxide, but, if possible, use a caoutchouc cork ; if not, give your cork a soaking in melted paraffin wax. Carefully pour down the thistle funnel dilute sulphuric acid (1:1); an action is at once set up, and the flask is filled by degrees with yellowish-green vapours, which pass over into the gas jar. Collect seven jars of the gas, and cover each jar with a well-greased cover-glass.
2NaCl + MnO2 + 2H2SO4 = Na2SO4 + MnS04 + 2H2O + Cl2
The preparation of chlorine should be conducted in a draught-chamber, as the inhaling of this gas causes irritation to the throat. If you do, by accident, happen to inhale any of it, pour a little alcohol on a piece of filter paper, place it over the nose and mouth, and breathe the vapour into the lungs. If the gas should slacken off coming over into the jar, warm the flask a little.
Chlorine Gas Laboratory Experiment I
Invert a jar of the gas over the hole in the tray of a pneumatic trough filled with water, and remove the cover-glass; the water gradually rises in the jar, showing the solubility of chlorine in water.
Laboratory Chlorine Gas Experiment II
Moisten a piece of madder-dyed red cloth with water, put it in a jar of the gas, and place the cover-glass again over the mouth of the jar ; the red cloth is gradually bleached.
Laboratory Experiment Chlorine Gas III
Pour a little concentrated sulphuric acid into another jar of the gas, and replace the cover-glass quickly, then shake the jar; after a short time put into the top of the jar a piece of dry madder cloth, similar to that used in the last experiment; it is not bleached. The gas in this experiment is dried by means of the sulphuric acid, and chlorine, when perfectly dry, does not bleach.
Chlorine Gas Laboratory Experiment IV
Take a small piece of filter paper, moisten it with turpentine, and plunge it into another jar of the gas; black smoke is at once seen in the jar, and a momentary flame; the filter paper is also charred.
C10H16 + 16Cl = 16HCl + 10C
Chlorine Gas Laboratory Experiment V
Take a little finely powdered antimony, and shake it slowly into a jar of the gas; the antimony sparkles and burns as it passes through the gas.
Sb + 3Cl = SbCl3
Chlorine Gas Laboratory Experiment VI
Place a piece of dry phosphorus in a deflagrating spoon, and pass it into a jar of the gas; the phosphorus takes fire, and burns with a brilliant flame.
P + Cl3 = PCl3
Laboratory Experiment with Chlorine Gas VII
Into the remaining jar of the gas put two pieces of paper, one with letters printed on it with printers’ ink, the other with letters written on it with ordinary ink; the printers’ ink remains unchanged, while the ordinary ink is bleached. Printers’ ink is essentially a mixture of carbon with some thickening material, while the ordinary ink partly consists of a vegetable substance containing a considerable amount of hydrogen in it.
Laboratory Experiment using Chlorine Gas VIII
Put a little very finely powdered iron pyrites in a test-tube with some water, and pass a gentle stream of chlorine through the liquid. Keep the test-tube shaken all the time, to maintain the particles of iron pyrites in suspension. The pyrites after a time disappears, and only a few white particles are left floating about.
FeS2 + 8H2O + 14Cl = FeSO4 +14HCl + H2SO4
2FeSO4 + H2SO4 + Cl2 = Fe2(SO4)3 + 2HCl
Filter the solution, and divide the filtrate into two portions. To the first portion add a little barium chloride; a dense white precipitate forms.
Fe2(SO4)3 + 3BaCl2 = Fe2Cl6 + 3BaSO4
To the second portion add a slight excess of ammonium hydrate: a foxy-red precipitate forms.
Fe2(SO4)3 + 6NH4OH = Fe2(OH)6 + 3(NH4)2SO4
Oxygen Derivatives of Chlorine
Use the apparatus employed in the preparation of chlorine, but with about half the quantity of manganese dioxide and sodium chloride in the flask. Make about 200 c.c. of a strong solution of potassium hydrate in a beaker, surround the beaker with cold water, and pass a gentle stream of chlorine through the solution. After about five minutes remove the beaker, pour half its contents into another beaker, then set it on some wire gauze on a tripod over a bunsen flame, and continue to pass the chlorine through
2KOH + 2Cl = KClO + KCl + H2O
the solution while it is boiling.
Take the cold portion of the potassium hydrate solution, notice its smell, it being different from that of chlorine, then perform the following experiments:
Laboratory Oxygen Derivatives of Chlorine Experiment I
Take a piece of madder-dyed cloth, and dip it into the solution; the cloth is not bleached.
Oxygen Derivatives of Chlorine Laboratory Experiment II
Pour a small quantity of the solution into a test-tube, and add a few drops of hydrochloric acid; you notice an action going on, and chlorine is evolved.
KClO + 2HCl = KCl + H2O + Cl2
Laboratory Experiment Oxygen Derivatives of Chlorine III
Take the piece of madder-dyed cloth used in Experiment I. and dip it again into the solution, then pass it quickly through a dilute hydrochloric acid solution, and wash it in water; it is now bleached. By the action of the hydrochloric acid on the potassium hypochlorite (KClO) contained in the pores of the cloth, chlorine is liberated, and as the cloth is moist, it is bleached.
Place the remainder of the cold solution of potassium hypochlorite into a retort, add a little very dilute nitric acid, and attach to it a small receiver, then apply a gentle heat to the retort; a colourless peculiar-smelling liquid condenses in the receiver.
KClO + HNO3 = KNO3 + HClO
Take now the boiling solution of potassium hydrate through which chlorine has been passing for some time, transfer it to a porcelain evaporating dish, and evaporate it down to about half its original bulk; then allow it to cool. Crystals of potassium chlorate (KClO3) separate out; drain off the liquid, and dry the crystals between pieces of filter paper.
6KOH + 6Cl = KClO3 + 5KCl + 3H2O
The potassium chloride (KCl) for the most part remains in solution, being more soluble than the chlorate.
Laboratory Experiment IV with Oxygen Derivatives of Chlorine
Take one or two of the potassium chlorate crystals you have prepared, place them in a test-glass, cover them with water; put into the glass also a very small piece of phosphorus, and set a thistle funnel in the glass, so that its lower end just touches the crystals, as shown in fig. 32. Pour a few drops of strong sulphuric acid down the funnel; a greenish gas is evolved, which attacks the phosphorus so violently as to cause it to take fire under water. This gas is the peroxide of chlorine (Cl2O4).
3KClO3 + 2H2SO4 = KClO4 + 2KHSO4 + Cl2O4 + H2O

INSTRUCTIONS TO THE STUDENT
The student has now obtained a fair knowledge of simple glass-working and of the preparation and properties of the more common gases. In the following sections he proceeds to examine solids and liquids (generally solids which are put into solution). First he examines an unknown substance by ‘dry tests’ for ‘ bases ’ and ‘ acids ’; the results so obtained are then confirmed by systematic ‘ wet tests.’
The order of work laid down is to be followed with most careful and patient attention to all details; the student has then done his part, but unless this is supplemented by equal care on the part of the demonstrator, the best results cannot be expected. The demonstrator, besides supervising the actual testing, must prepare a carefully graded series of substances leading from simple salts containing one ‘base’ and one ‘ acid ’ to complex mixtures containing four or five ‘bases’ and several ‘ acids ’ (including insolubles). The substances must all be carefully selected with the definite object of teaching the student some important point in every mixture he analyses. Indiscriminate preparation of mixtures leads to waste of time and bad work.
In his first tests the student may be given salts of known composition, and his work is then checked by unknown salts from the demonstrator’s set. For instance, on the next page he may take ZnCl2, SnCl2, Pb, Bi2S3, and so on for practice, and when fairly confident, his proficiency or otherwise is checked on ‘unknown’ salts given by the demonstrator.
