Table of Contents
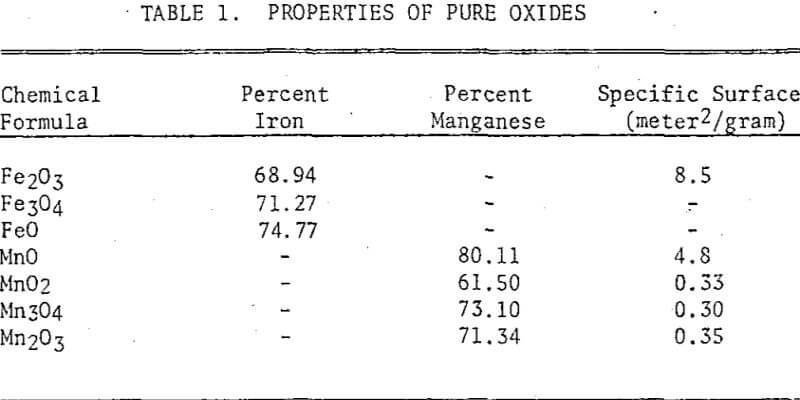
The chlorination behaviors of pure iron and manganese oxides were investigated by combining a thermogravimetric analysis (TGA) technique with batch-boat roasting followed by leaching. Ferrous and manganous oxides could be chlorinated readily, but, in the absence of a reductant, the higher oxides of both iron and manganese were difficult to chlorinate. Thermogravimetric analysis curves were drawn to illustrate the complexities of the reactions, and the possible mechanisms were discussed. Then three manganiferous materials from the Cuyuna Range of Minnesota were treated by a process involving the selective chlorination of manganese followed by leaching. The results were interpreted in the light of the chlorination mechanisms observed on the pure iron and manganese oxides.
Materials
The pure ferric oxide used in the study was of analytical grade. Magnetite and wustite were prepared from the pure ferric oxide by heating it for one hour at 900°C in a mixture of CO and CO2. For the magnetite a CO-CO2 ratio of 1:10 was used, and for the wustite 1:1 was used. Pure manganous oxide was prepared by decomposing manganous carbonate of analytical reagent grade in a nitrogen atmosphere at 500°C. Manganese dioxide of analytical reagent grade was used as purchased. Manganese tetroxide was prepared by heating the manganese dioxide for several hours at 1000°C. Manganic sesquioxide was prepared by heating the manganese dioxide to 600°C. T
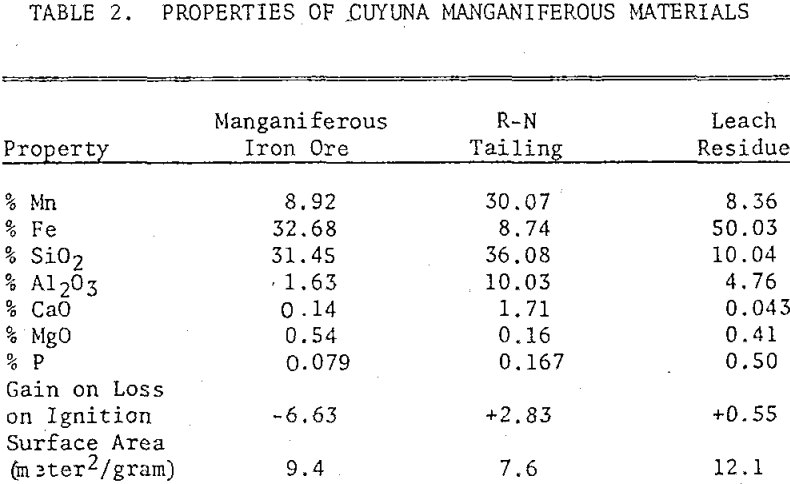
Three manganiferous meterials from the Cuyuna Range were also used in the study: a manganiferous iron ore, an R-N process tailing, and a leach residue of an R-N tailing. The manganiferous iron ore consisted mainly of hematite, magnetite, goethite, and quartz with minor quantities of stilpnomelane, minnesotaite, and chlorite. The manganese minerals identified were manganite and a minor quantity of pyrolusite. The ore was ground in a pulverizer to minus 100 mesh prior to chlorination.
Procedures
A thermobalance was constructed using a tube furnace, a quartz tube with gas inlet and outlet arrangements, and a quartz sample holder suspended via a quartz helical spring. The weight change was detected with an optical reader mounted alongside the reaction tube. The sensitivity of the thermobalance was approximately 0.3 mg per scale division. The furnace was regulated to give a constant rate of heating of 10°C per minute. Nitrogen gas was washed with pyrogallol-potassium hydroxide solution, and dried through a calcium chloride, tower. Chlorine gas was used after drying through calcium chloride. A few additional tests, particularly those in the absence of the reductant, were later carried out with a similar thermobalance at the Resources Research Institute, Kawaguchi, Japan. This thermobalance had a heating rate of 6°C per minute and a sensitivity of 0.5 mg per scale division.
A sample and the activated charcoal were mixed in a predetermined molar ratio, and the mixture in the range of 0.25 to 0.40 gram was weighed in the quartz sample holder. After the holder had been placed in the thermobalance, the reaction tube assembly was purged with nitrogen.
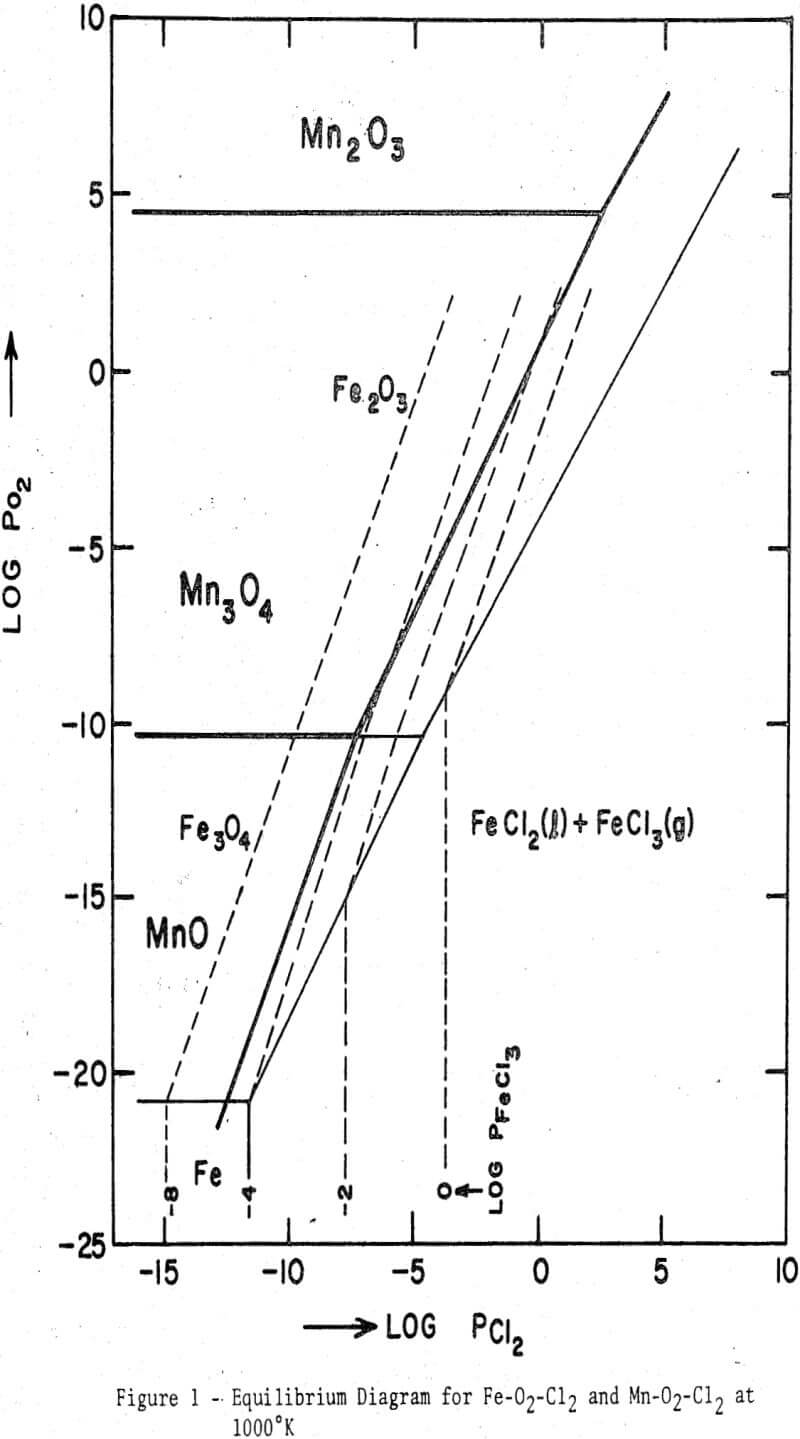
An equilibrium diagram showing the stability regions of the oxides and chlorides of both iron and manganese has been calculated from the free energy data. It is evident that iron oxides can be readily chlorinated at an intermediate temperature when the oxygen partial pressure is low, e.g., in the presence of a reductant. When a reductant is absent, oxygen released as a result of the chlorination reaction may impart a certain oxygen partial pressure, and at sufficiently high partial pressures the chlorination reaction may become unfavorable. During the chlorination of magnetite or wustite the oxygen may be consumed by the sample to produce higher oxides, thereby influencing the subsequent chlorination behavior.
Fe2O3 + 3 Cl2 = 2 FeCl3 + 3/2O2……………………………………………….(1)
3 Fe3O4 + 3/2 Cl2 = FeCl3 + 4 Fe2O3…………………………………(2)
4 FeO + 3/2 Cl2 = FeCl3 + Fe3O4…………………………………….(3)
Fe2O3 + 3C + 3Cl2 = 2FeCl3 + 3CO…………………………………………(4)
Fe2O3 + 3C + 3Cl2 = 2FeCl3 + 3/2 CO2…………………………………………..(5)
The major variables affecting the chlorination reaction are temperature, chlorine partial pressure and flowrate, carbon content, and reaction time. The individual effects of these variables and their interactions were investigated by thermogravimetric (TGA) analysis and batch-boat experiments combined with leaching.
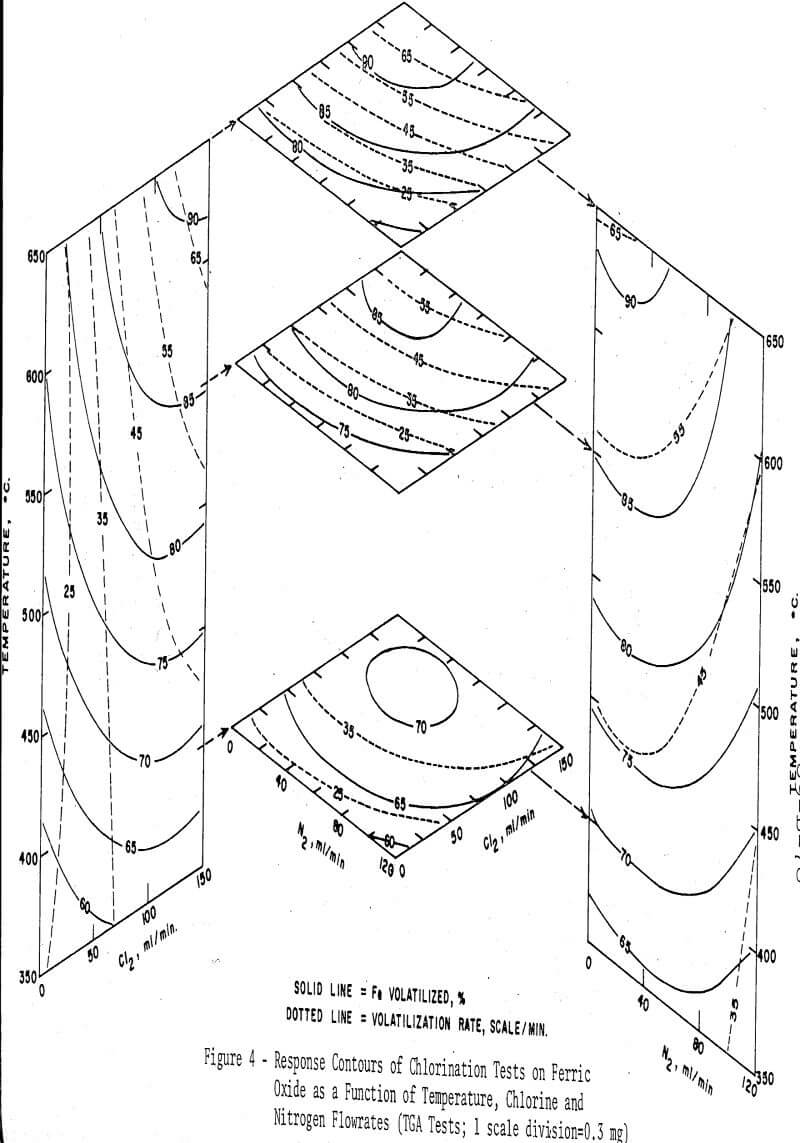
To investigate the effects of temperature, and of the flowrates of chlorine and nitrogen on the reaction rate and on the maximum extent of conversion into ferric chloride, a three-factor orthogonal composite design was used and the experimental points were fitted with a second-order regression equation by the method of least squares. As before, the amount of the charcoal addition was fixed at two equivalents. The reaction time was not controlled, but each test was continued until the weight of the sample reached a constant value. The regression equations were solved with a digital computer to obtain points for plotting response surfaces. The response surfaces are presented in Figure 4. It is readily seen in the figure that the volatilization rate is uniquely dependent on the flowrate of chlorine, but relatively independent of temperature. The total weight change, or the percentage of iron volatilized, however, is noted to be more strongly dependent on the temperature. The flow-rate of nitrogen significantly lowers both the volatilization rate and the percentage of iron volatilized when its flowrate exceeds about one half that of the chlorine, due presumably to the lowered partial pressure of chlorine in the flowing gas.
Chlorination of Manganese Oxides
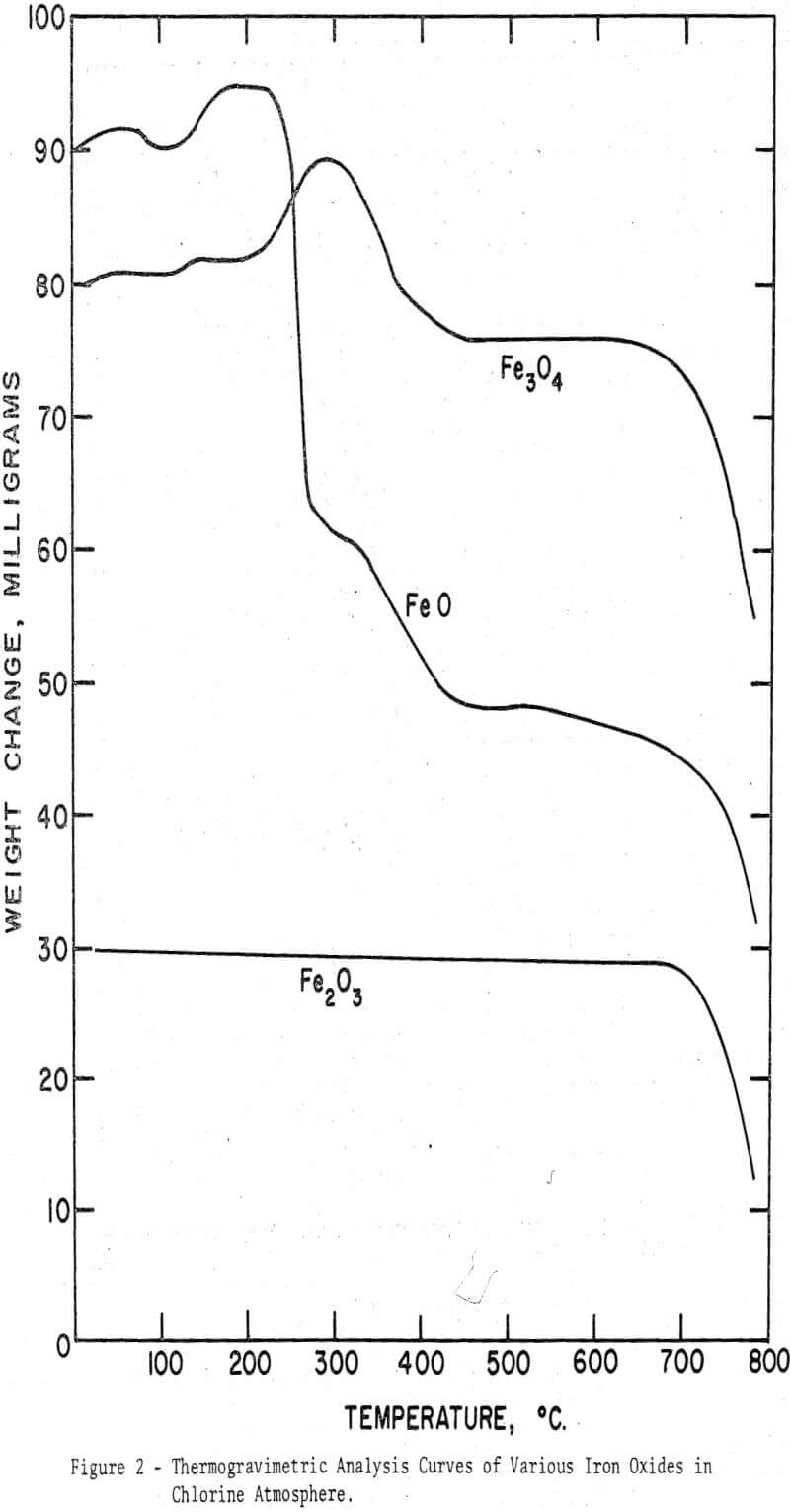
From the foregoing observations on the chlorination behavior of various iron oxides it became apparent that the higher oxides were more difficult to chlorinate than the lower oxides. Only in the presence of carbon could ferric oxide be readily chlorinated at relatively low temperatures. To investigate if a similar generalization might be extended to manganese oxides, the four oxides, MnO, Mn3O4, Mn2O3, and MnO2, were chlorinated in the thermobalance, initially in the absence of carbon. Evident in the figure is the relation between the order of magnitude of the weight gain and the oxidation states. A reliable estimate of the extent of the conversion to manganese chloride, however, cannot be made since the fraction of oxygen released as gas and reacted to produce higher oxides is unknown. The interpretation of the TGA curves may be further complicated by the thermal decomposition of MnO2 to Mn2O3 between 650° and 700°C, as reported from differential thermal analysis curves.
2 Mn2O3 + Cl2 = MnCl2 + 3 MnO2
Mn3O4 + Cl2 = MnCl2 + 2 MnO2
MnO + Cl2 → MnCl2 + MnxOy
MnO + ½C + Cl2 = MnCl2 + ½CO2
MnO2 + C + Cl2 = MnCl2 + CO2
Mn2O3 + 3/2 C + 2Cl2 = 2MnCl2 + 3/2 CO2
Mn3O4 + 2C + 3Cl2 = 3MnCl2 + 2CO2
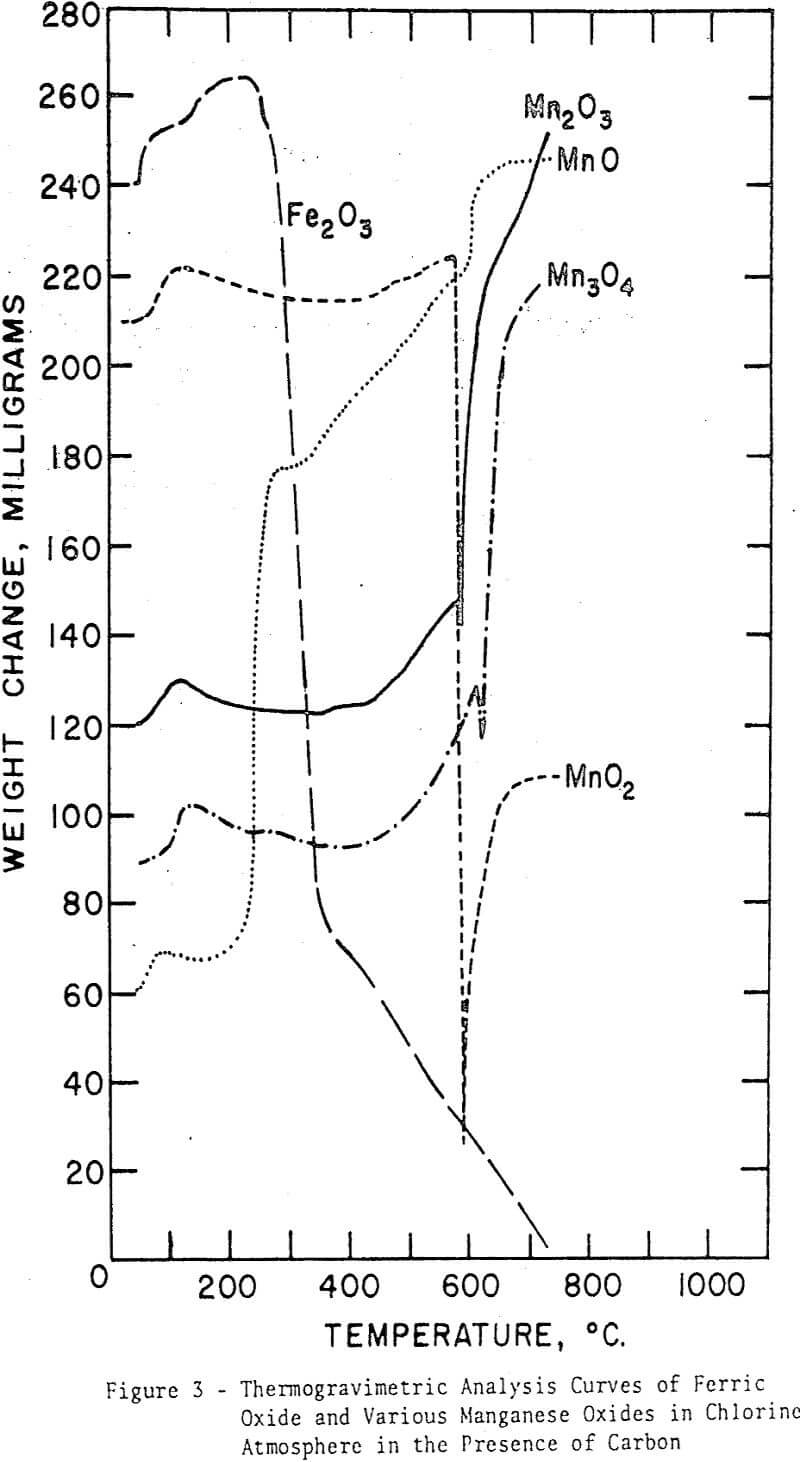
For each oxide, an initial weight increase, similar to that for ferric oxide, is evident. This increase may be attributed to the adsorption of chlorine on charcoal. The curve for manganous oxide shows several distinct stages of chlorination reaction similar to those for the same oxide in the absence of carbon. The first stage is, however, more pronounced, whereas the third-stage increase is markedly less, but more rapid, clearly indicating the effect of a reductant on the overall reaction rates. The other three oxides show certain common features. Lack of the first-stage reaction is evident; the temperature must be too low for the carbon to exert any influence on the oxidation state of the manganese oxides. The second-stage weight increase of intermediate velocity starts at about 440°C (similar to the curves for Mn3O4 and Mn2O3 in the absence of carbon) and ends at about 600°C, where a sudden decrease in weight is observed in all cases. At this point the chlorination reaction is greatly accelerated. Since it is known that MnO2 decomposes to Mn2O3 between 650°C and 750°C and that this temperature range may be lowered appreciably in reducing atmospheres, the sudden decrease in weight near 600°C may be related to the decomposition of MnO2.