Table of Contents
The fact that chloride oxidates can solubilize base metals from sulfide concentrates has been known since the 1880’s. The work of Hoepfner in 1902 was particularly accurate for its time with regard to the chemistry of chloride solutions. Recent work has dealt with the understanding of chloride extraction thermodynamics by modelling the behavior of metal chloride complexes
BHAS initially developed a chloride/sulfate leach for processing a copper sulfide byproduct from lead smelting. A 5 k ton per year copper production plant is being installed to handle the copper/lead matte. The process has been tested on chalcocite and chalcopyrite concentrates. Testing has been performed in a pilot plant capable of producing 250 kg per day of copper.
Process Description
The oxidative leach is performed at atmospheric pressure at 85 degrees Centigrade with both chloride and sulfate ions. The copper is recovered into a sulfate solution by solvent extraction with a chloride compatible reagent. The copper is subsequently electrowon from the sulfate solution. Copper recovery is estimated at 98 percent.
The process incorporates four main steps;
- A first stage leach at 103-107 degrees Centigrade which solubilizes approximately half the copper and produces a discharge solution with the copper present primarily as cuprous ions. Leaching is performed with cupric and ferric chloride.
- Preparation of cell house feed by reaction with cement copper.
- Recovery of copper as granules in diaphragm cells while simultaneously oxidizing copper and iron for recycling to second stage leach.
- Complete digestion of copper minerals in second leach utilizing raffinate. The second leach is performed at 150 degrees Centigrade under an oxygen environment. Excess iron is also purged at this stage.
Cyprus Metallurgical has been the most open of all companies with descriptive data on their process. The leach consists of two stages, both operated at atmospheric pressure and at 95 to 105 degrees Centigrade. Copper is recovered from solution by crystallization of cuprous chloride at approximately 35 degrees Centigrade. The cuprous chloride is then reduced with hydrogen gas to produce elemental copper and hydrochloric acid. The acid is recycled and the copper refined to remove silver and silica (added in the hydrogen reduction step). The leach solution is regenerated with oxygen during which the excess iron in solution precipitates as jarosite.
The Dextec process for copper concentrates has progressed to the extent that a continuous pilot plant is being constructed in South Africa. The pilot plant will contain ten Dextec cells. Insufficient details have been published to determine if the pilot plant will produce copper or simply remove contaminants from a copper concentrate.
Both the copper and lead processes incorporate leaching of sulphide concentrates in diaphragm electrolytic cells. For the lead concentrate, the leaching is conducted in a hydrochloric acid/sodium chloride brine. A concentrate slurry is maintained at the bottom of the circular cell without allowing anode contact. As described, the process does not solubilize copper or zinc and would produce a lead powder containing one percent impurities. The cells are built in 10-12k liter sizes and multiple units are installed to give the desired capacity. Lead recoveries of 97 percent are reported.
The ELKEM process for treating dirty sulfide concentrates is rather a complex process. The process consists of the following steps:
- Counter current leaching
- Lead recovery by crystallization of lead chloride
- Zinc recovery by solvent extraction with tributyl phosphate (TBP), pregnant solution purification and electrowinning in diaphragm cells.
- Copper recovery from chloride solution by electrolysis in diaphragm cells.
- Regeneration of the solution with the subsequent purging of iron as akaganeite and carphosiderite.
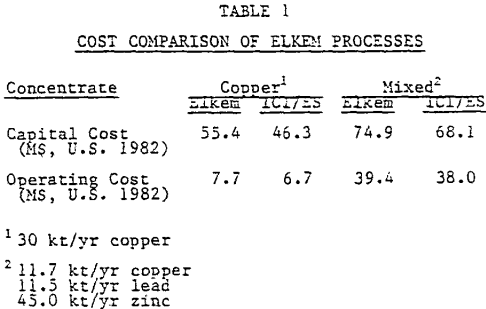
The ELKEM/ICI process is a modification of the ELKEM process. The new process has not undergone the same degree of testing as the original ELKEM process, but only sufficient testing to allow for the completion of feasibility studies. The new process has replaced the original ELKEM process due to its more favorable economics.
The Sherritt Gordon-Cominco process involves a single stage leach at approximately 100 degrees Centigrade utilizing cupric chloride, ferric chloride and chlorine. The cuprous chloride is recovered from the leach discharge solution by the injection of butadiene gas. The resulting complex salt is decomposed by heating the solution under vacuum. Copper is recovered from the resulting high purity cuprous chloride by: 1) oxidizing the cuprous chloride to copper oxvchioride, 2) hydrolysis of the oxychloride to CuO with caustic and 3) hydrogen reduction of the copper oxide. The leach solution is regenerated with oxygen with the subsequent purging of excess iron as ferric hydroxide.
Application of Chloride Hydrometallurgy
Most developers of a chloride leach process see their process as the ultimate replacement for smelting. After being involved in chloride leaching off and on for twelve years, the author takes a somewhat narrower view of the potential for chloride hydrometallurgy. In the negative view, chloride leach processes can not economically compete against smelters for the treatment or pure, single component concentrates.
The potential for chloride hydrometallurgy lies in the following areas:
- Processing of low grade bulk concentrates
- Upgrading of concentrate prior to pyrometallurgical processing
- Treatment of low quality sulfide concentrates containing large quantities of impurities and precious metals.
Materials of Construction
- Polypropylene, Kynar and Teflon lined pipe, tanks and valves.
- fiber reinforced plastic tanks and pipe.
- Acid brink lined autoclaves.
- Titanium and titanium/palladium clad autoclaves.
- High Temperature (80 degrees Centigrade) rubber linings.
- Composite plastics pumps and valves
- epoxy coatings for concrete and steel.