The chemical alteration of water by acid mine drainage begins with the exposure to the atmosphere of pyrite associated with coal during the mining process. The exposed sulfide minerals, which are relatively insoluble in their original state, are converted by oxidation to soluble sulfuric acid, which in turn dissolves other minerals containing manganese, aluminium and calcium.
FeS2 + 3O2 + 2H2O → 2H2SO4 + Fe +³
The resulting ferric iron subsequently reacts with water to form a ferric hydroxide precipitate, often referred to as “yellowboy”, that settles out on stream beds.
Fe +³ + 3H2O → Fe(OH)3 + 3H+
Experimental
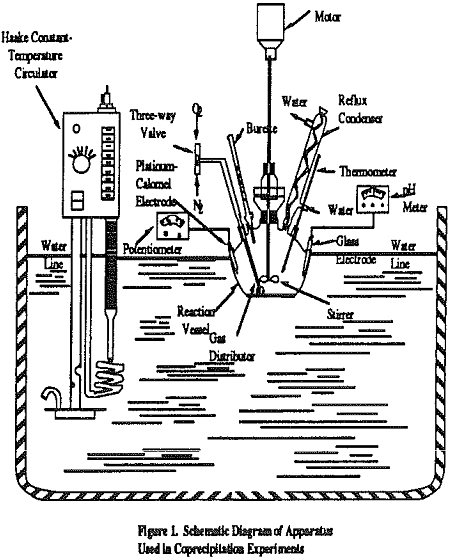
After addition of the solution to the reaction vessel, nitrogen was bubbled into the solution while it was being stirred at 540 r.p.m. to remove the dissolved CO2 and O2. Afterwards, desired amounts of FeSO4 .7H2O was added to the solution. Then, the pH was adjusted by adding 4N NaOH or Ca(OH)2 solution and kept constant throughout the experiment. The suspension was oxidized by passing the air. In the former case, the ORP value increases rapidly up to nearly 0 MV from -700 MV at the end of the oxidation reaction. The Iron – oxide precipitate thus obtained was allowed to undergo sedimentation for 20 minutes after the rapid change of the ORP value. The resultant sludge was dried in air and resuspended in water. The solution was used for magnetic separation to obtain ferrite fraction.
Results and Discussion
When divalent Fe ions, (Fe²+) coexist with non- ferrous divalent metal ions, is an aqueous solution, the addition of an equivalent amount of alkali will induce the following reaction.
Mx ²+ + Fe²+ 3-x + 6(OH)- → Mx Fe 3-x (OH)6………………………………………(1)
Mx Fe 3-x (OH)6 + ½O2 → Mx Fe 3-x (OH)4 + 3H2O……………………………………..(2)
Ferrite can also be produced by adding alkali to an aqueous solution in which trivalent iron Fe+³ ions, coexist with divalent metal ions at a ratio of 2:1.
While temperature is very important for the second step of the reactions. Figure 2 shows the effect of temperature on the ferrite formation. With the temperature below 40°C, large portions of FeOOH and 5- FeOOH are formed.

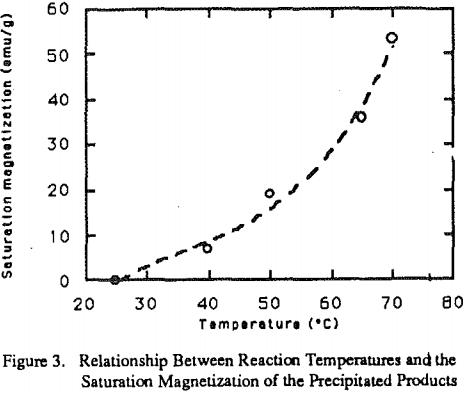

The final products with this method demonstrated very good magnetic properties. Table 1 shows the efficiency of heavy metal removal and properties of the final products obtained from conventional and modified methods for the simulated wastewater. Table 2. lists the results using modified ferrite process for real acid mine water, and subsequent dissolution tests.