Table of Contents
Tests run on using organics to influence the fracture strength of solids gave an almost bewildering array of results on the four substrates. Two general classes of tests were carried out:
1) a procedure consisting of introducing a notch in a small rectangular sample, placing this sample in an environmentally controlled chamber for humidity, chemical dosage control, etc., applying tension to the sample, and finally measuring the rate of notch propagation: and 2) the addition of water, chemicals, etc., to an eight inch ball mill and running tests on standard feed material for specified periods of time.
It was with the third concept, that is, controlling the slurry viscosity (rheology) that the greatest initial effects of chemistry on grinding rates were observed. It was on this concept that a very extensive chemical screening program was carried out leading to the filing and granting of a series of chemical use patents on grinding additives by Manfroy and Klimpel (1978-79). The initial premises behind this approach were: 1) that as one starts from rather poorly packed slurries (e.g., <30-40% solids by volume) and gradually increases the solid packing per unit volume (e.g. by increasing the % solids and/or adding fines) the ability to grind faster (e.g., finer) in a tumbling media mill increases; 2) that this effect will continue as long as the viscosity does not get so high as to dramatically change the nature of the media to slurry contact in the tumbling media device; and 3) the use of appropriate chemicals to control (maintain or lower) slurry viscosity with this increasing packing efficiency will promote faster grinding rates over those rates that are possible with changing water/solid ratios only (hence act as a grinding aid).
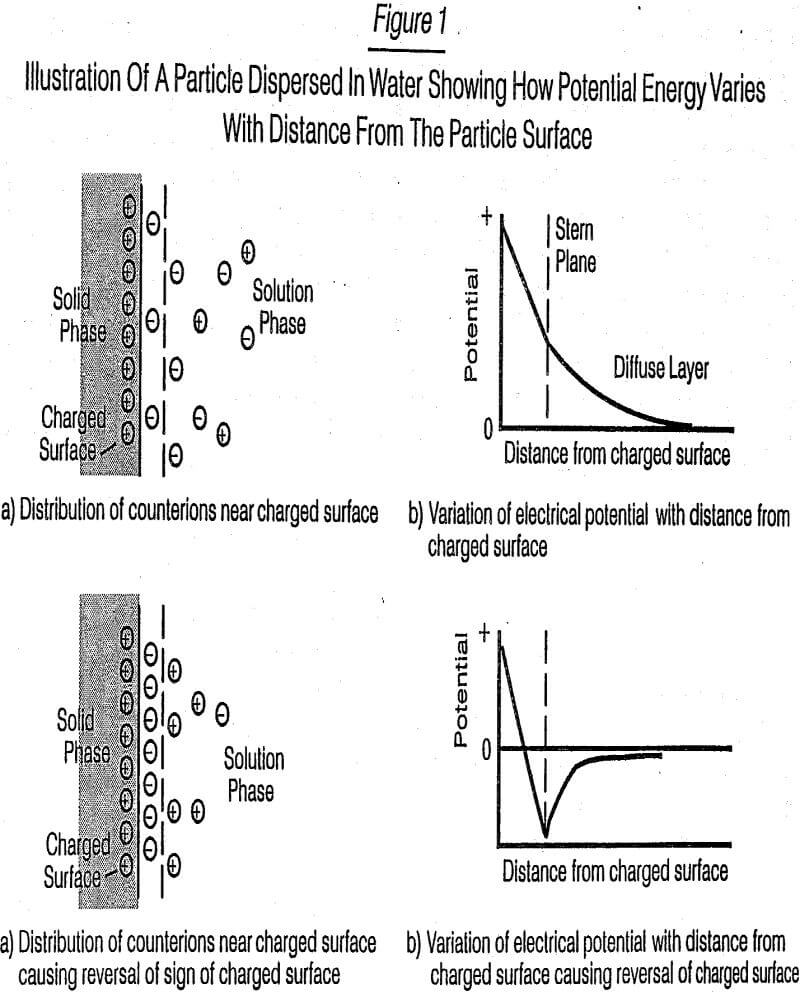
Discussion of Dispersion Theory of Solids in Aqueous Slurries
In an aqueous environment, when the surfactant is dissolved, the lyophobic (hydrophobic) group causes a distortion of the water with a resulting increase in the free energy of the system. This implies that less work is needed to bring a surfactant molecule than a water molecule to solid surfaces. Thus the surfactant concentrates at the surfaces. Conversely, the presence of the lyophilic (hydrophilic) group(s) prevents the surfactant from being totally forced from the solvent (water) as a separate phase. In this study, because water is a highly polar solvent, the hydrophobic group for both technical and economic reasons consisted of a hydrocarbon mass (backbone) of suitable size. Because of the surface charge nature of the mineral substrates, the surface-active portion(s) of the agents to be studied were primarily anionic in nature, that is, the agents bear a negative charge.
Once an aqueous mixture of the mineral ore particles is prepared, a variety of individual events can occur. Medium-sized particles, e.g., 100 µm in diameter, will settle in a few seconds by gravity (i.e., Stokes Law) unless agitation is present or the slurry is very thick [contains a yield value as described in Klimpel (1982 and 1982/83)]. Larger particles, e.g., 1000 µm, will settle very quickly, possibly oven with severe agitation or being present in a very thick slurry. Surfactants have little direct effect on either of these particle sizes. It is with the smaller particles (<50 µm and certainly less than 10 µm) that surfactants play an effective part in controlling slurry viscosity or dispersion (i.e., controlling the resistance of flow of the slurry as a function of energy input to move the slurry). A significant portion of this viscosity control mechanism is related to preventing agglomeration (coagulation) of small particles which lead to the formation of larger particles. As just discussed, these larger particles cannot be manipulated as easily either by increasing energy input or by using surface-active agents.
In addition, there were other factors that came strongly into play in the chemical agent screening program. The first is the fact that as the number of hydrophobic (charged) groups per molecule increases, there is usually an
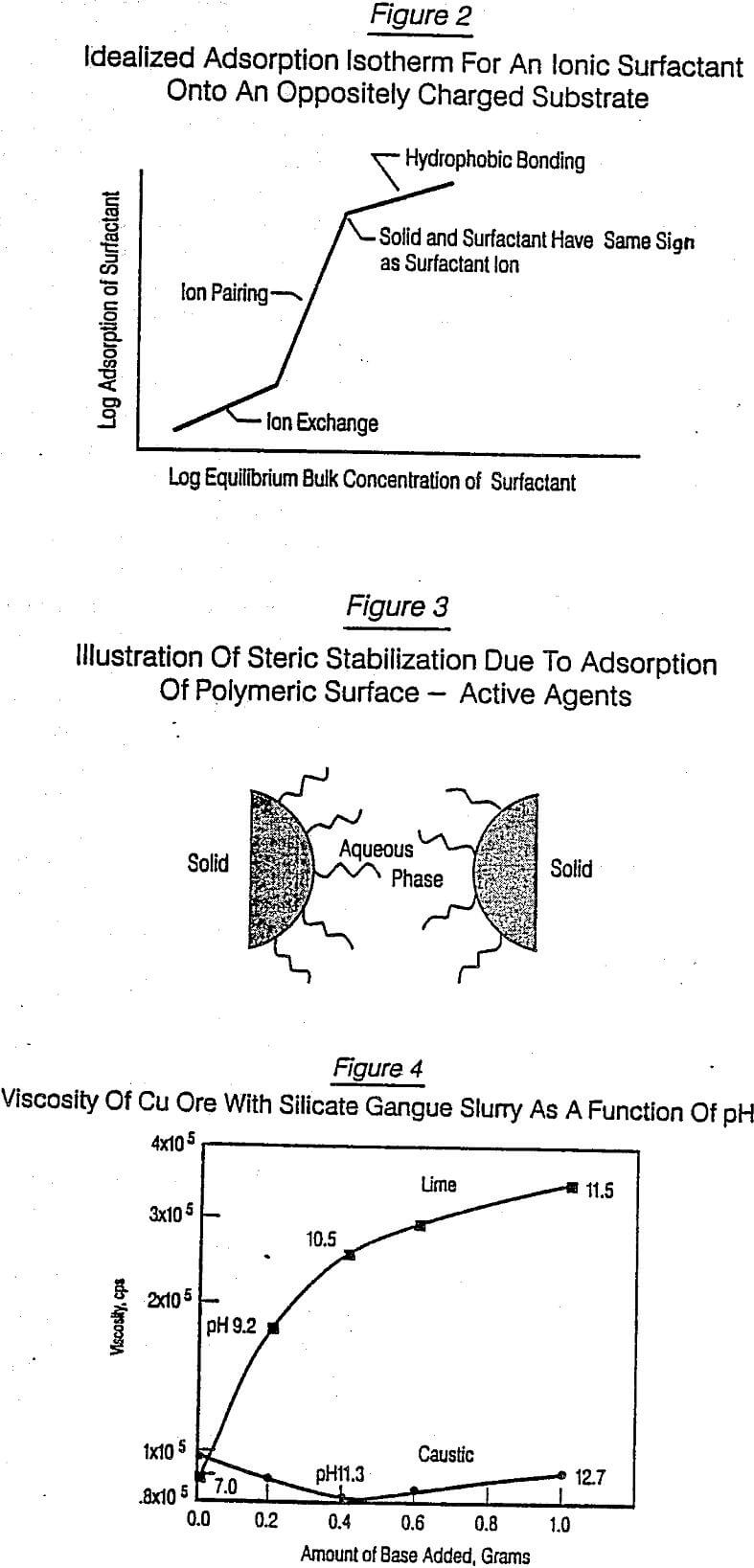
increase in the agent’s solubility in water. This often causes a decrease in its adsorption onto a particle surface, depending on the strength of interaction of the agent and the particle surface under a particular set of conditions, i.e., pH, electrolyte concentration. Thus, it is not uncommon for a particular type or class of agent chemistry to exhibit a maximum in dispersion or viscosity control capability with increasing number of ionic groups per unit length of the agent. Thus, the molecular density of the charged groups of the agent had to be optimized.
Some Results of Screening Appropriate Chemical Reagents
It is not possible in a single paper to summarize all of the data generated and the structural trends identified with the reagents screened during the period 1973 through 1979. A great deal of chemical polymer experience, synthesis, and property-structure development work was involved in this study. Over several hundred reagents were evaluated with those chosen, Manfroy and Klimpel (1978-79), for optimal performance being represented by families of partially water soluble polymers or copolymers of anionic monomers where the preferred anionic groups were carboxylate or sulfonate on a polyethylene hydrophobe backbone. The molecular weight distributions ranged from 200 to 100,000 with typically the optimal range for viscosity control being 2000 to 20,000 depending on the specific reagent.
The standard test procedure used was to grind 500 grams of solid with just enough water to form a viscous slurry calculated to give a T-bar Brookfield viscosity between 100,000 and 150,000 cps. Then one cc increments of water are added and the change in viscosity is measured. This curve then represents the base against which the effect of chemical additives is compared. The additives were prepared as 10% active solutions by weight so that each cc of additive solution represented a treatment level of 0.2 mg agent/g of dry ore (or 0.4 pounds per ton of ore). The chemical solution is added in increments until 5 cc (2.0 pounds per ton of ore) is achieved. Again the viscosity change is plotted as a function of treatment level.
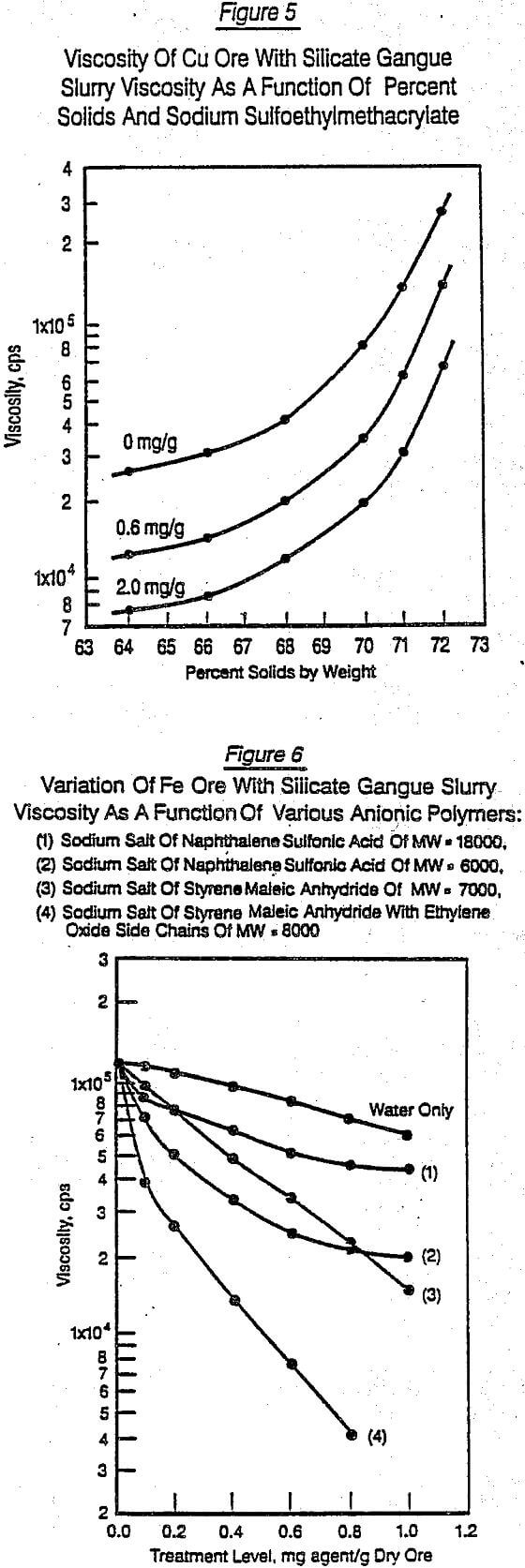
As has been emphasized in previous publications, e.g. Klimpel (1982/83), for a fixed size distribution, there often is a relatively small region where very large changes in viscosity result from small changes in the percent solids. It is, of course, also the region where there is the greatest advantage in increasing throughput if the corresponding slurry viscosity can be lowered at any given percent solids setting. Figure 5 gives a typical result of such a test run on a siliceous copper ore in this critical region as a function of varying dosages of sodium sulfoethylmethacrylate (average molecular weight, MW, of -9000). From such data, the relative effectiveness of each specific chemical structure with regard to dosage could easily be made. During this screening phase, the viscosity control differences between various chemical agents tested, holding all the ore parameters constant, ranged from increasing viscosity over the 0 mg/g case by 50% to lowering the 0 mg/g case by 99%. Again, the interpretation of this type of viscosity data in forms of breakage rates has been previously documented, Klimpel (1982, 1982/83) and will not be repeated in this paper.
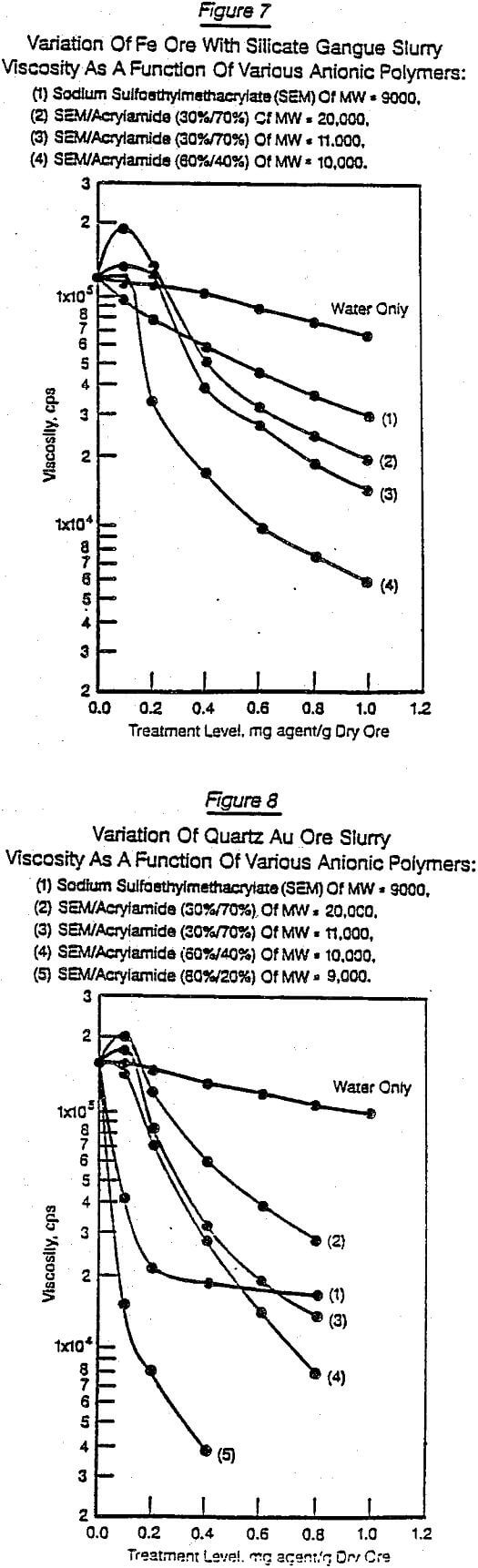
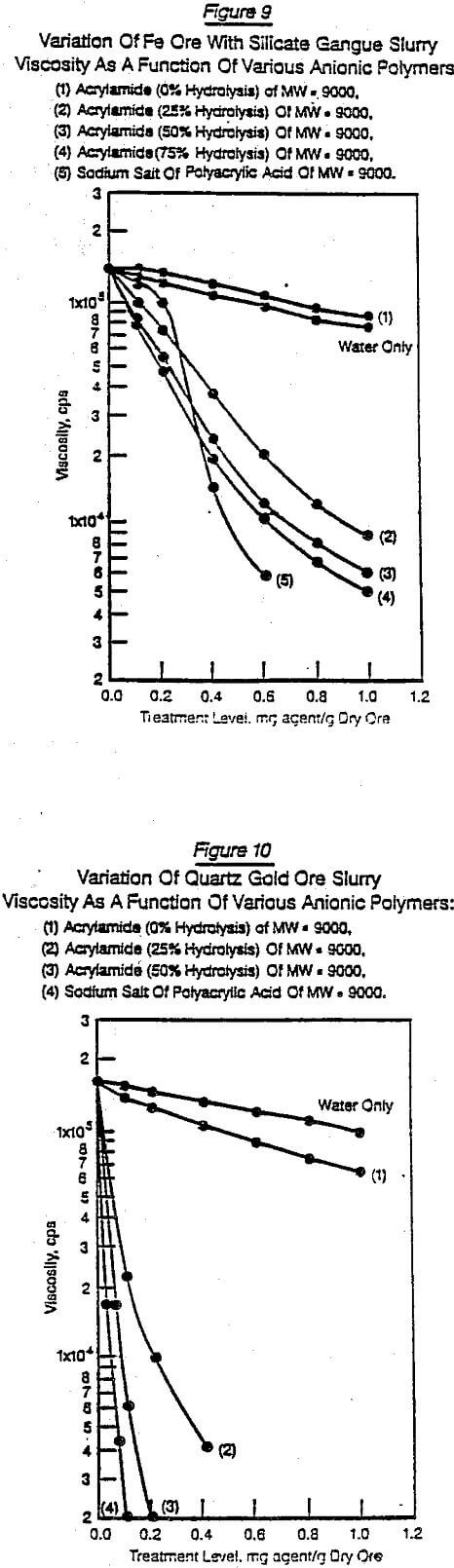
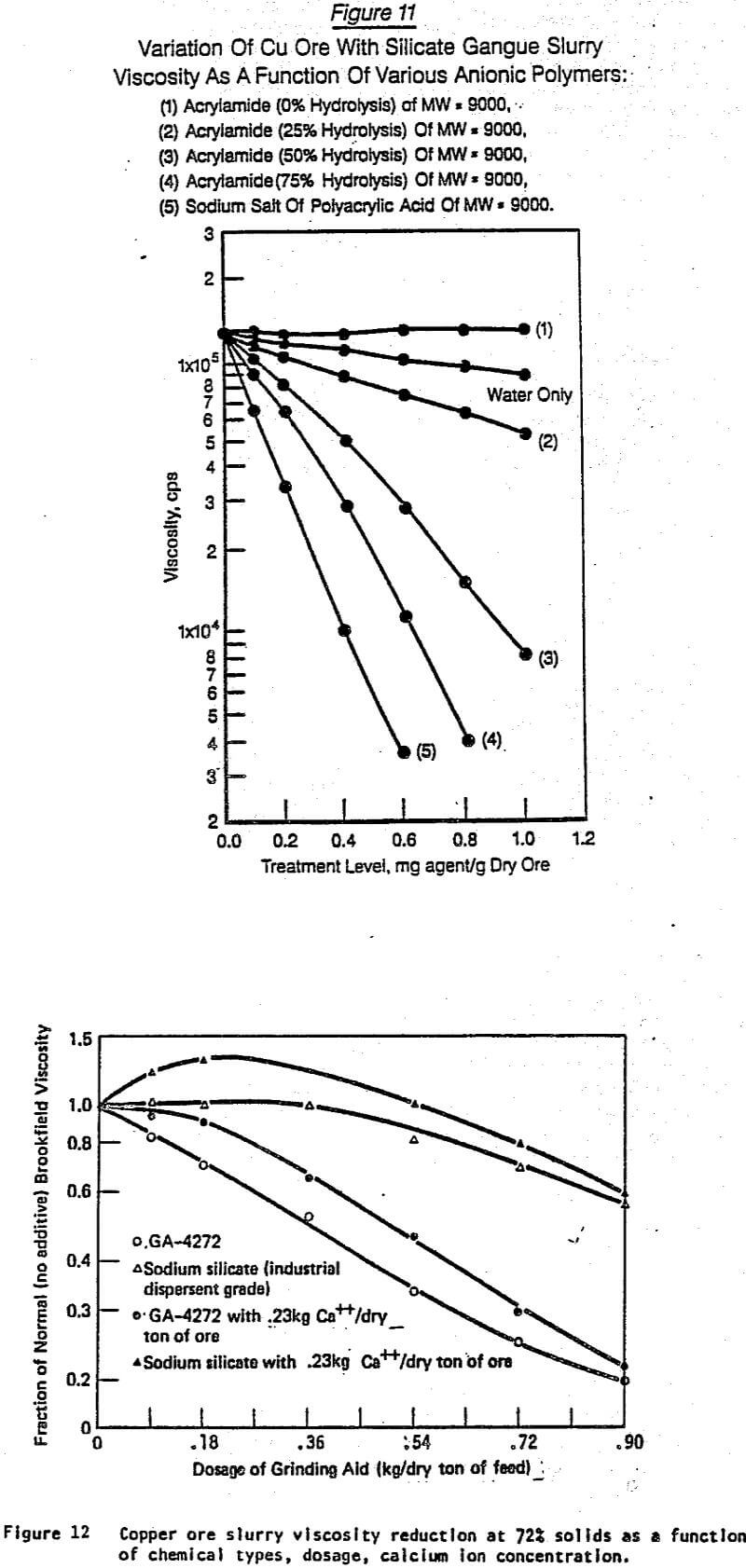
Figures 6 through 11 were selected from the large number of chemical structure/performance trends generated. They illustrate typical differences due to changes in chemical structure, molecular weight, dosage, and the nature of the solid substrate. Figures 6 and 7 show on the same substrate (siliceous iron ore) the difference between four polymer structures [naphthalene sulfonic acid, styrene maleic anhydride, sulfoethylmethacrylate (SEM), and SEM/acrylamide copolymers]. In addition, Figures 6 and 7 show how selected changes within each polymer family can significantly influence the performance character of that general chemical structure.