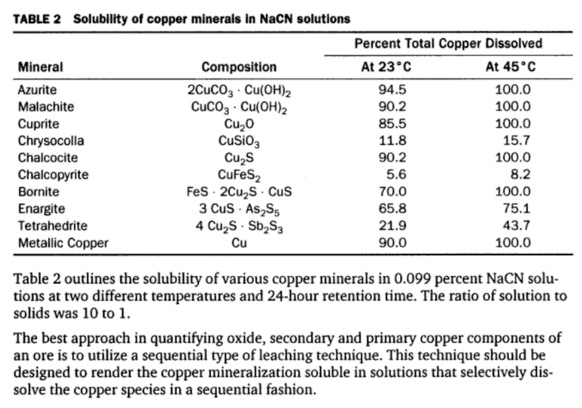
In discussing the Reaction Products from Copper Minerals and Cyanide you will find that when an excess of copper mineral is acted upon by cyanide solution, dissolution of copper continues at a decreasing rate until equilibrium is established and no more copper goes into solution. The rate will vary with the particular mineral used as shown by the data in Table 2.
 When the excess mineral is filtered off and the solution analyzed for copper and total cyanide by distillation, the molar ratio of NaCN to Cu is usually between 2.5 and 3 to 1. This indicates the presence of both NaCu(CN)2 and Na2Cu(CN)3 with a preponderance of the latter. As mentioned previously, some authorities claim that at least one other complex, Na3Cu(CN)4, in this series exists, in which the molar ratio is 4 to 1. If this complex exists, then it has appreciable solvent effect on copper minerals, otherwise, equilibrium would not be established at a considerably lower molar relationship.
When the excess mineral is filtered off and the solution analyzed for copper and total cyanide by distillation, the molar ratio of NaCN to Cu is usually between 2.5 and 3 to 1. This indicates the presence of both NaCu(CN)2 and Na2Cu(CN)3 with a preponderance of the latter. As mentioned previously, some authorities claim that at least one other complex, Na3Cu(CN)4, in this series exists, in which the molar ratio is 4 to 1. If this complex exists, then it has appreciable solvent effect on copper minerals, otherwise, equilibrium would not be established at a considerably lower molar relationship.
Other products are formed when copper minerals are dissolved in cyanide depending on the particular copper mineral treated. With oxidized minerals containing cupric copper, some cyanate is formed and with sulphide minerals some thiocyanate is formed.
Some have consider that in solutions containing copper and thiocyanate the copper exists as copper thiocyanate (Cu2(CNS)2) dissolved in cyanide. Some authorities claim that the complex Cu2(CNS)2*6 NaCN exists.
Malachite and azurite, in which a large proportion of the copper is in the form of cupric carbonate, react with cyanide solutions in much the same manner as a cupric salt. That is, cupric cyanogen complexes are first formed which then break down to the cuprous form, liberating cyanogen. This cyanogen reacts with alkali to form cyanide and cyanate.
The reactions as regards cupric carbonate may be expressed as follows:
2 CuCO3 + 8 NaCN = 2Na2Cu(CN)3 + 2Na2C03 + (CN)2
(CN)2 + 2 NaOH = NaCNO + NaCN + H2O
The overall equation being:
2 CuCO3 + 7 NaCN + 2 NaOH =
2 Na2Cu(CN)3 + 2 Na2C03 + NaCNO + H2O
Cyanidation researchers treated a sample of chalcocite with a cyanide solution containing 0.472% NaCN and 0.009% NaOH. After 24 hours agitation in a wide-mouthed bottle on rolls, the excess chalcocite was filtered off. An analysis of the filtrate showed it to contain 0.202% Cu, 0.432% total NaCN by distillation, 0.015% NaOH, and 0.070% NaCNS equivalent. The molecular relationship of total NaCN to Cu was 2.78 to 1. Only about 9% of the total NaCN was converted to thiocyanate. Thus, even if all of the thiocyanate were in the form of copper thiocyanate, the amount would only be a small percentage of the total copper in solution. The greater part of the copper, therefore, was probably in solution as sodium cuprocyanides. The reaction might be represented approximately as follows:
2 Cu2S + 11 NaCN + 2.5 O2 + H2O =
2NaCu(CN)2 + 2Na2Cu(CN)3 +
NaCNS + Na2S04 + 2 NaOH
The solution was titrated with silver nitrate, the first appearance of opalescence being taken as the end-point. The amount of NaCN indicated by this titration was 0.129%. When this amount is deducted from the total NaCN as determined by distillation, i.e. 0.432%, the relationship between the remainder, 0.303% NaCN, and the copper in solution, 0.202%, corresponded closely to the complex NaCu(CN)2; the titration, therefore, measured all of the sodium cyanide present in excess of this complex.
When the same solution was titrated with silver nitrate using 0.5 grm. of potassium iodide as indicator, the titration represented only 0.004 percent NaCN. Weighed amounts of sodium cyanide were added to portions of the solution which were titrated in the same manner. It was found that the silver nitrate-potassium iodide titration did not measure fully the sodium cyanide increments made to the solution. For example, when 0.05% solid NaCN was added to the solution titrating 0.004% NaCN, it would be expected to titrate 0.054% NaCN. Instead, it titrated only 0.038% NaCN. It was not until sufficient sodium cyanide was added to the solution to increase the molecular ratio of total NaCN: Cu to 4: 1 that the increase in titration corresponded to the cyanide addition. Apparently the additions of sodium cyanide were combining with the copper in solu¬tion to form finally the complex Na3Cu(CN)4. The amount of cyanide thus indicated by the silver nitrate-potassium iodide titration is probably not a measure of the true free cyanide strength of the solution but a measure of the amount of cyanide which can ultimately dissociate from the sodium cuprocyanides present.
