Table of Contents
Chemical mining is the in situ extraction of metals from ores located within the confines of a mine (broken or fractured ore, stope fill, caved material, ores in permeable zones) or in dumps, prepared ore heaps, slag heaps, and tailing ponds on the surface. These materials represent an enormous, untapped, potential source of all types of metals. The field of chemical mining, now in its infancy, encompasses the preparation of ore for subsequent in-place leaching, the flow of solutions and ionic species through rock masses and within rock pores, the leaching of minerals with inexpensive and regenerable lixiviants under prevailing conditions of the in-place environment, the generation and regeneration of such solutions, and the recovery of metals or metal compounds from the metal-bearing liquors.
It is not inconceivable that eventually our ore reserves will consist largely of low-grade, refractory, and inaccessible new deposits and low-grade zones near previously worked deposits, caved and gob-filled stopes, waste dumps, tailing ponds, and slag heaps. Chemical mining promises economic recovery from these types of deposits. Before it can come of age, however, a much better understanding is needed concerning its chemical and physical aspects.
Heretofore, this kind of mining has been more or less limited to the extraction of copper from low-grade materials. It has a much greater potential than this. Practically all metals are susceptible to leaching in the in situ environment. Processes will soon be developed for the in situ extraction and recovery of metals such as copper, lead, zinc, nickel, manganese, uranium, silver, gold, molybdenum, and mercury.
Any process used in mining or mineral processing has certain advantages and disadvantages. A few for chemical mining are listed below:
Advantages
- Chemical mining can often be used to recover metals economically from materials that could not be so treated by more conventional mining, milling, and smeltering processes.
- A chemical mining plant usually requires less capital investment than a mine and mill plant.
- A chemical mining process usually increases a mine’s ore sources and reserves. Low-grade or inaccessible ore zones, gob and caved fill, and dumps and tailings may become ores.
- The leach liquors obtained through chemical mining usually lend themselves to a variety of metal recovery processes. The pure metal or metal compounds so obtained may be of greater value to a mine owner than the sulphide or oxide products normally obtained by conventional milling processes.
- Chemical mining may prove applicable to recovering metals from ores that are too refractory for conventional processes.
- Chemical mining can often be used in conjunction with a conventional mining or milling process to boost metal recoveries and increase ore reserves.
Disadvantages
- Both physical and chemical restrictions may limit the usefulness of a chemical mining process. The effectiveness of contacting ore with solutions and the recovery of leach solutions from fee system without appreciable loss are two important physical factors. Dissolution or dissolution rates, metal precipitation, and solution regeneration chemistry are major chemical factors.
- Testing a chemical mining process short of actual field operation sometimes proves difficult.
- Ground-water contamination may result from some chemical mining operations.
- We presently lack basic information on the physical and chemical factors involved.
Chemical Mining Technology
The field of chemical mining can be divided into (1) mining economics and ore evaluation, (2) elements of the leaching phase, (3) preparation of ores, (4) practical aspects of in situ-leaching, (5) reagent generation and regeneration, and (6) recovery of metals from leach liquors.
Mining Economics and Ore Evaluation
In considering the economic exploitation of a deposit through chemical mining, one must determine the size of the deposit, tonnage of ore in place, and amount of metal contained therein. In past as well as present mining operations, the cut-off grade has been governed by the total operating cost, including mining, which usually constitutes a significant portion of the over-all cost. However, in chemical mining considerations, the cost of mining would be minor and, therefore, the cut-off grade would be lowered correspondingly. This lowering would inevitably increase the tonnage as well as the metal content of the deposit, which in turn would influence the over-all economics of the venture.
Unfortunately, the literature does not have much information available concerning the relationship between tonnage and grade. No doubt records of mining companies may contain such valuable information, and some attempts should be made to obtain pertinent data from these sources.
Lasky, Musgrove, and a few others have studied this relationship through a statistical analysis of known deposits and perusal of past records of some mining companies. These studies reveal that there is an exponential relationship between grade and tonnage of ore reserves. Especially for deposits in which there is a gradation from relatively rich to relatively lean material, there appears to be a consistent mathematical relation between tonnage and grade, according to the equation
G = K1 – K2 log T………………………………………………………………(1)
where T is the tonnage produced to a given time plus the estimated reserves, G is the weighted average grade of this tonnage, and K1 and K2 are constants to be determined for each deposit. Using equation (1), Lasky showed that for a typical porphyry copper deposit, the tonnage increases at a compound rate of 14. 9 per cent for each 0.1 per cent decrease in grade.
Our studies using the published data on ore grades and reserves revealed the following information for copper (porphyry), molybdenum (hydrothermal disseminated replacement or vein type), and uranium (secondary redistributed ore):
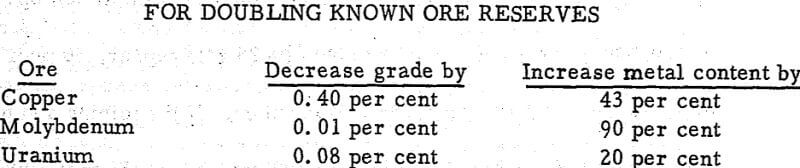
These data indicate that the known reserves and metal contents may be increased significantly through corresponding lowering of the grades of the deposits. Chemical mining schemes allow such lowering of grades while increasing tonnages and metal contents.
Another important aspect of chemical mining that needs further scrutiny is determining a minimum reserve and grade of a deposit for profitable exploitation. Hardly any data are available on this subject. The only guide line worthy of consideration is the operational data from copper dump leaching and in-place leaching practices in which the grade of material treated is above 0.16-per cent.
The important factor in chemical mining, as in dump leaching, is making sure that the major portion (+90%) of the specified volume of leach solution fed to the deposit or dump is recovered with a given minimum amount of metal content in solution over the life of the economic operation. This minimum metal content in the specified volume is such that the value of the recovered metal will provide for the cost of operation, amortization, and profit.
Naturally, the metal content in leach solution differs from metal to metal, depending on its price. From a hydrometallurgical recovery viewpoint alone, it is estimated that at the current prices of metals and operating conditions, the break-even contents, for a minimum operation of 200, 000 gallons a day are, 250 ppm (0. 25 g/l) copper, 50 ppm (0.05 g/l) molybdenum, and 10 ppm (0.01 g/l) uranium. If the mining and development cost, overhead, and profit amount to 200 per cent of the metallurgical treatment cost, then the metal contents in leach solution must be 750 ppm (0.75 g/l) copper, 150 ppm (0.15 g/l)molybdenum, and 30 ppm (0.03 g/l) uranium for an economic operation.
In general, it may be safe to assume that because of lower treatment and capital costs incurred in chemical mining, one could economically treat a sufficiently large deposit containing half the grade of deposits currently mined and milled. Thus, deposits containing 0.25 per cent copper, 0.12 per cent molybdenum, and 0.1 per cent uranium could profitably be mined with this technique. In actual practice, it may well be possible to treat even lower grade deposits than these.
Let us emphasize, however, that utilizing chemical mining schemes would require a change in our outlook on all phases of the mining operation, especially in exploration, reserve estimation, and over-all evaluation and consideration of newer parameters in leaching and metal recovery.
Elements of the Leaching Phase
Accessibility, physicochemical interaction, and transport constitute the elements of the leaching phase involved in chemical mining. Limitations imposed on any of these factors restrict the leaching process.
Accessibility is essential because interaction between the desired constituents and the lixiviant cannot take place in the absence of contacts, which depends on exposure and penetrability. The factors to consider are locations of the values, their volume and shape distribution, exposure area, specific surface, particle size, porosity, capillary pressure, viscosity pressure, solubility of gases in lixiviant, and surface roughness.
Physicochemical interaction converts the desired constituents from a fixed to a mobile condition and is governed by the solubility of the solid in leach solutions and vapor pressure in gases. Knowledge of free energies of reactants and products helps to determine whether a reaction is possible. The kinetic factors involved include time, concentration, diffusivity, specific rate constants, and wettability.
The first two elements by themselves do not ensure successful leaching without transport of products away and reactants to the zone of interaction through diffusion and convection. Diffusion is governed by concentration gradient and diffusivity, which in turn are influenced by particle size, micropore radius, temperature, and molecular mass. On the other hand, convective flow concerns interparticle penetration and is restricted by pressure gradient, permeability, viscosity, and surface roughness.
Broadly speaking, the factors governing leaching can be grouped into two major classifications; namely, physical and chemical. The majority of the leaching studies in the past have emphasized chemical factors. However, it is essential that we consider the physical factors also, since they definitely influence the leaching process. We must develop new techniques for physical and chemical testing of ore samples and for establishing the limitations of and determining optimum parameters for successful extraction of values from the broken ore.
Preparation of Ores
To be processed by chemical mining techniques, ores are in place and require fragmentation prior to leaching, in place and permeable enough to permit flow of solutions through them, or previously mined or fragmented. Waste dumps, tailings, filled stopes, and caved ground fall into the last group.
Several means have been proposed for fragmenting an ore body prior to in situ leaching. In recent years, various authors have proposed the use of nuclear explosives. Griswold suggested using hydrofracting techniques to break ore for subsequent leaching. As envisioned by him, liquid explosives would be injected into ore bodies along planes of weakness and detonated at a slow rate.
Although conventional mining methods have seldom been used to prepare ore for in situ leaching, there is no reason that they could not be used. Present methods like caving techniques and shrinkage stoping adapt well to breaking ore for subsequent underground leaching.
In some instances, it may be advantageous to use a conventional mining method for selectively removing the higher grade ores from an ore body prior to in situ leaching of the lower grade materials. As an example, if, in the mining of an ore body by a shrinkage method, a lower grade zone adjacent to the higher grade body were to be drilled as the stope progressed upward and the holes were loaded as the stope was drained, the low-grade material could be broken into the stope cavity and then leached.
Varying degrees of preparation may be required when the ore is already broken. When it is located underground as stope fill, very little ore preparation is required. Ores to be treated by chemical mining techniques on the surface, however, may or may not require some preparation prior to leaching. Waste dumps, slag heaps, and the like may require crushing and stacking on prepared pads, whereas fine materials like tailings may require rebedding, slime removal, or placement on an impervious pad.
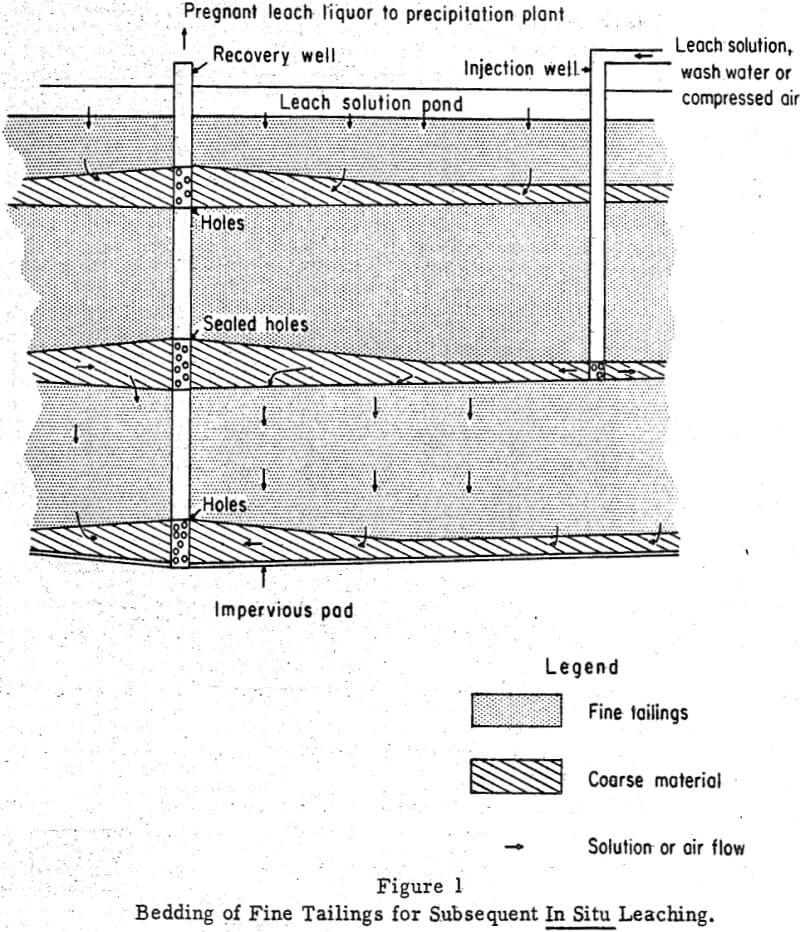
Figure 1 illustrates how fine tailings might be leached by downward percolation techniques. In this system, an impermeable pad of plastic, asphalt, or concrete would prevent solution losses through ground seepage. Alternating layers of coarse rock and fine mill tailings would then be laid down over the pond area. Leaching, either concurrent with tailings deposition or following deposition, would be by downward percolation through the tailings beds of limited thickness. Percolation rates would be considerably higher through beds of limited thickness than unlimited ones. Solutions could be fed on the top bed or injected to selected beds through wells. After percolating through the tailings, the pregnant leach solutions would flow to a central recovery well. Possibly, gases could be injected to displace solutions and to react with the metal-bearing minerals.
Practical Aspects of In Situ Leaching
In chemical mining, large volumes of ore contact relatively large volumes of leaching solution over a period of time. The mechanics or pattern of solution flow varies according to the chemistry involved, the means available for solution containment and recovery, and the need to prevent ground-water contamination.
Two principal types of solution-flow through a porous ore bed are downward percolation under the influence of gravity and flow within an immersed system.
In a downward percolation system, leach liquor is usually distributed over the top of a pile of ore and allowed to flow through the pile to a liquor collection system. This type of solution-ore contact has the advantage that only the floor of the ore bed need be impervious to the leach solutions. This type of flow allows some circulation of air within the ore bed, possibly an important factor in the oxidation of ore minerals. Use of downward percolation in an underground ore bed could prevent solution seepage into the ground-water strata. The chief disadvantage of this type is that incomplete solution-ore contact can result from the impermeability of local zones and from channeling.
Downward percolation has been, and probably will continue to be, the principal method of leaching ore beds. This technique has been discussed by several authors. It has been used in the leaching of waste dumps crushed and uncrushed ores on prepared pads, mill tailings and filled and caved workings. Solution transfer may be either by convection or by diffusion. Convection may be caused by mechanical means or by differences in the density of the solution at different points within the system. This type of flow offers positive movement of solutions through an ore bed at a desired flow rate and complete contact of the solution with the bed. The main disadvantages of this type of flow are the necessity of having the ore in an impermeable container to prevent solution loss and contamination of ground water, the restriction of natural oxidation by air circulation, the necessity of pumping solutions, and the large amount of solution involved in the leaching system.
Immersion techniques have been used in chemical mining. Copper oxide-sulphide ores have been leached in concrete tanks by upward percolation techniques at Inspiration for many years. Utah Construction Company recently conducted leaching tests on an unmined uranium ore body by using a series of injection, monitor, and recovery wells to force a leaching solvent through the permeable uranium ore body and to recover the pregnant leach solutions. Pirson proposed similar techniques for the in situ leaching of phosphate beds. Uranium is being recovered from mine waters at Grants, New Mexico. Copper has recently been recovered from water in the flooded Rio Tinto mine, Mountain City, Nevada.
Certain features inherent to an underground environment can aid leaching. One is the hydrostatic pressure imposed on an immersed deposit at depth and another is the natural increase in rock temperatures with depth. If gas(es) were introduced into an inflowing stream of solution entering a deposit under a hydrostatic pressure and/or the rock temperatures, were above the normal temperatures at the surface, one could possibly use this system as a huge, low temperature-high pressure autoclave. Leach reaction rates can usually be increased many fold when temperatures and gas pressures are increased.
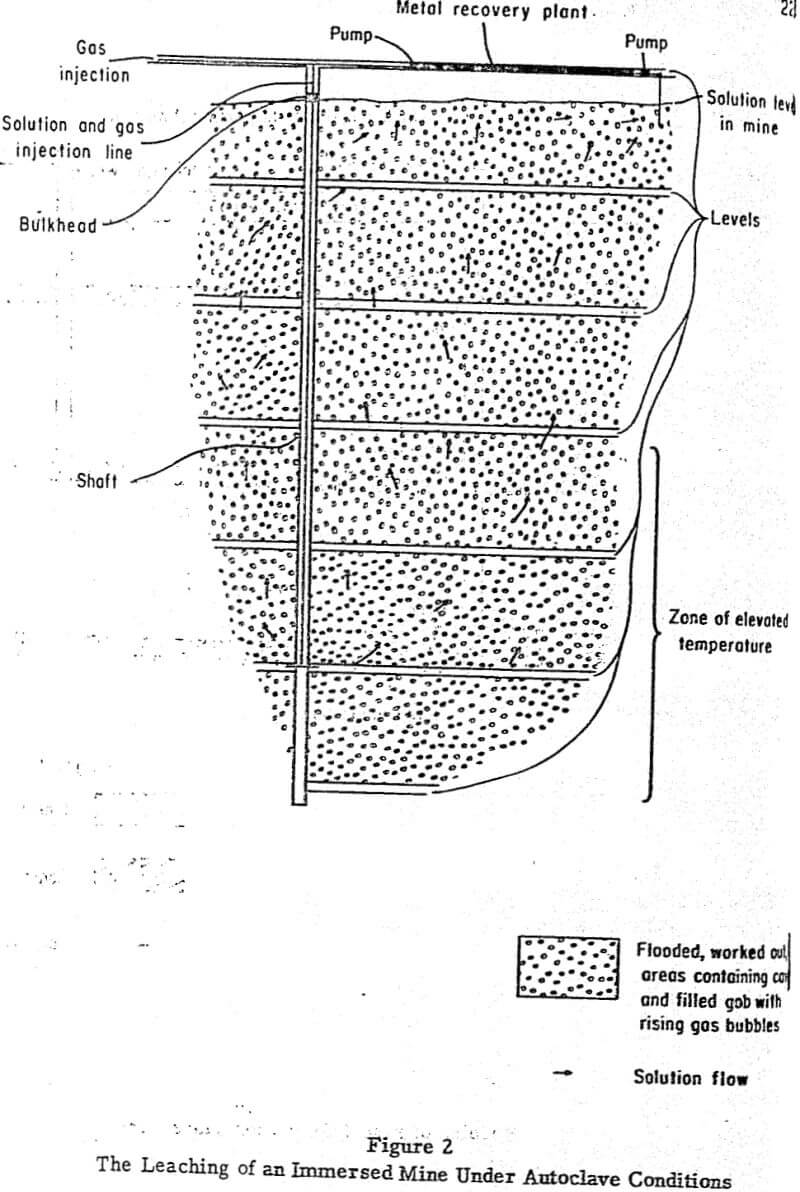
Figure 2 diagrammatically illustrates how autoclave conditions might be imposed on an immersed mine. This system consists of an abandoned mine flooded with a leaching solution. A pipe introduced into the bottom of the mine via a shaft would carry a gas (usually air) and/or spent leach liquor into the bottom of the system. These conditions would greatly enhance the rates of dissolution of most oxide and sulphide ore minerals. Solution circulation within the system would result from the air-lift and convection effects of rising gas bubbles, convection currents caused by the variation in temperature between the top and bottom of the system, and pumping of solution in closed circuit within the system. Metal would be recovered from the pregnant solution in its circuit from the top to the bottom of the mine.
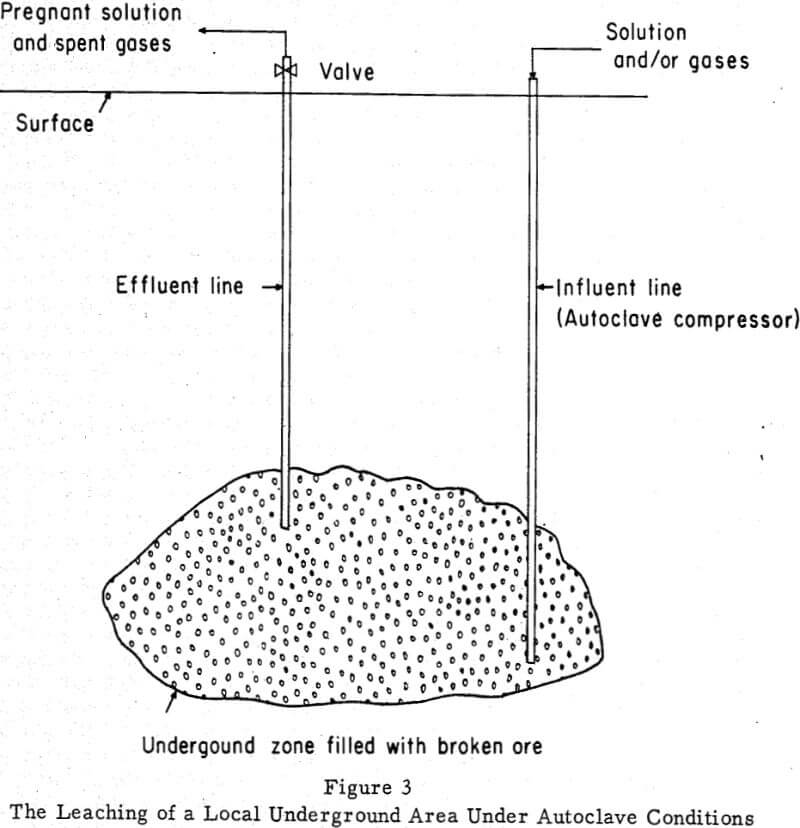
A similar technique could leach a particular area underground only the leaching zone would need immersion. If the area constituted a worked-out part of the mine, bulkheads placed in appropriate drifts or openings would seal it off. If it were a fragmented or permeable area underground reached by boreholes (Fig. 3), no seal would be required. Solution and/or gases would enter the leaching zone under pressure and either would be forced back to the surface by the internal pressure in the system or would require pumping. If the volume of the cavity leached were not too large, heating the influent leach solution could prove advantageous.
If the effluent stream of gas and solution discharges from the leached zone at a lower pressure than the influent stream, one could introduce a low-pressure gaseous stream at the top of the influent pipe and allow the downward-flowing liquid to compress the gases during their flow. The downward-flowing stream would act as a hydraulic compressor and as an autoclave. Possibly, a hydraulic compress or autoclave of this type would serve to oxidize or chemically change the leaching solution prior to its use in either a percolation or immersion type of system.
Another idea that may warrant consideration is that the flow of a low-amperage current could be directed from an electrode on one side of a broken ore zone undergoing leaching to another electrode on the other side. Under given conditions of voltage and amperage, one might change the leaching solutions chemically, accelerate physicochemical reactions, and cause an increased rate of ion migration to a local area of solution recovery.
Solution Generation and Regeneration
Inasmuch as the chemical reagents used in chemical mining generally constitute a major cost item and greatly influence leaching, reagent generation and regeneration is a very important part of any chemical mining process. Some reagents can be generated and regenerated by natural processes in the leaching cycle, whereas others require various chemical processes.
Practically, the only time a leach solution is generated and regenerated by natural processes is in the production of sulphuric acid and ferric sulphates from pyrites and spent ferrous sulphate leach liquors under air oxidation conditions. Although this process is used in leaching great quantities of copper from both sulphide and sulphide-oxide copper ores, very little is really known about the reaction mechanisms; reaction rates and factors influencing them, such as and as an autoclave. Possibly a hydraulic compressor-autoclave of this type would serve to oxidize or chemically change the leaching solution prior to its use in either a percolation or immersion type of system.
Another idea that may warrant consideration is that the flow of a low-amperage current could be directed from an electrode on one side of a broken ore zone undergoing leaching to another electrode on the other side. Under given conditions of voltage and amperage, one might change the leaching solutions chemically, accelerate physicochemical reactions, and cause an increased rate of ion migration to a local area of solution recovery.
Solution Generation and Regeneration
Inasmuch as the chemical reagents used in chemical mining generally constitute a major cost item and greatly influence leaching, reagent generation and regeneration is a very important part of any chemical mining process. Some reagents can be generated and regenerated by natural processes in the leaching cycle, whereas others require various chemical processes.
Practically, the only time a leach solution is generated and regenerated by natural processes is in the production of sulphuric acid and ferric sulphates from pyrites and spent ferrous sulphate leach liquors under air oxidation conditions. Although this process is used in leaching great quantities of copper from both sulphide and sulphide- oxide copper ores, very little is really known about the reaction mechanisms; reaction rates and factors influencing them, such as liquid ammonia. Much research work needs to be done to determine how these materials can be used efficiently.
Our current studies will determine the effectiveness of common leaching reagents, such as acids, acid-iron salts, and salts like NaCl, Na2CO3, NaHCO3, in leaching ores of copper, lead, zinc, nickel, silver, gold, uranium, and molybdenum under chemical mining conditions. These tests include percolation leach conditions, static leach conditions, and high gas pressures-low temperature (below 100° C) conditions. A large column-type autoclave maybe used soon to leach coarse materials under each of these conditions.
The Recovery of Metals from Leach Liquors
The last phase of any hydrometallurgical process, including chemical mining, is the recovery of metals from leach liquors. Conventional purification of a metal-containing solution followed by recovery of metals or compounds from the solution by either chemical or electrolytic precipitation is employed to obtain the marketable product. This recovery technique is adequately covered in the literature and its effectiveness is clearly demonstrated in several successful plant practices.
In connection with chemical mining applications, however, the recovery phase poses certain technical problems that may influence the over-all effectiveness of the process. One such difficulty concerns treating a large volume of very dilute metal-bearing solutions that may contain more than one valuable metal. Unlike copper, not all metals easily precipitate on scrap iron. This may require recirculation of the leach solution to build up the metal content and then bleeding off of a small part of the concentrated leach stream for metal recovery.
Newer techniques of ion exchange, solvent extraction, and charcoal sorption may effectively concentrate dilute leach liquors. These procedures have proved very effective for processing large volumes of leach solutions containing more than one valuable metal. The Climax process for recovering oxide molybdenum values by charcoal sorption and the New Mexico Bureau of Mines procedure recently developed for recovery and selective separation of molybdenum, tungsten, and rhenium by sorption processes are typical of techniques that metal recovery systems will increasingly employ.
Since the crux of the chemical mining process is the particular lixiviant in leach solution, any recovery phase that regenerates the solvent or provides an essential component of the leach solution would be the preferrable procedure. Also, since chemical mining depends on the continuous circulation of the leach solution at its peak volume, it is imperative that the retention time in the metal recovery step be as short as possible. This requirement necessitates a metal recovery procedure that is relatively quick and capable of handling large volumes effectively.
Remarks
We have attempted to proffer a novel approach for extracting metal values contained in low-grade deposits, worked-out mines, dumps, and tailing piles. With the ever increasing demand for today’s metals, the necessity of treating complex and low-grade ores, increasing operational costs, and the public awareness of environmental pollution factors, future metal production will inevitably employ chemical mining on an increasing scale. The scope of this mining method encompasses interdisciplinary science and technology, requiring application of the principles of basic sciences, economics, mining, metallurgy, hydrology, and allied disciplines. Although some technological information comes from several dump- and a few in-place leaching operations for copper and uranium, we do not know enough about this technique.
Some researchers have tried to develop newer metal recovery techniques, but a much more concentrated endeavor would take fullest advantage of the chemical mining process for extracting metal values from marginal, low-grade deposits, dumps, and tailings.
The dearth of information illustrates the need for broad and imaginative research. Inasmuch as in-place leaching occurs on coarse materials on a large scale over a period of time and under conditions considerably different from most leaching practices, it behooves us to study the process from both basic research and practical application standpoints.