A suitable material for the student to operate on is the well known brand —Portland Cement. As the student has already examined in detail a silicate, the following notes are given somewhat briefly. A good cement should consist chiefly of SiO2 and CaO, with a little Al2O3 and Fe2O3, and less than 2% MgO, and 1.5% CO2 and SO3. From this the student will see that the accurate determination of MgO, CO2, and SO3 is a matter of much importance, and that in many cases the determination of these three items may be sufficient to decide the fate of the cement. The student, however, is advised to work out the complete scheme, as a check is thus obtained on the accuracy of his work.
SiO2.—Take 1.5 gms. finely powdered cement and boil in a porcelain evaporating basin with 50 c.cs. aqua regia. Evaporate to dryness. Add 100 c.cs. HCl (5E.). Boil and filter into a 500 c.c. flask. Wash well, allowing washings to come up to the mark. Reserve the solution, which contains Fe2O3, Al2O3, CaO, MgO, K2O, Na2O, SO3.
Dry the residue, and fuse with five times its weight of a mixture of K2CO3 and Na2CO3 in a platinum crucible. Cool, dissolve, and treat as before described for SiO2. Reserve the solution, which contains some Al2O3.
Al2O3,Fe2O3.—Precipitate the Al2O3 in this last solution by means of NH4HO. Filter, wash, dry, ignite, and weigh.
Agitate the solution in the 500 c.c. flask. Transfer 300 c.cs. to a beaker. Render alkaline with NH4HO. Boil, filter, and wash. Redissolve and repeat precipitation. Reserve the filtrates. The precipitate consists of Al2(HO)6; and Fe2(HO)6. Separate and estimate the Fe2O3 and Al2O3 as before. Calculate back from 300 c.cs. to 500 c.cs. Add the Al2O3 to that obtained in the filtrate from the SiO2, and the total Al2O3 and the Fe2O3 are thus obtained.
CaO.—To the filtrate from the iron and the alumina add (NH4)2C2O4 in slight excess. Allow to stand for a few hours. Filter and wash with dilute NH4HO (E.). Redissolve and repeat precipitation. Reserve the filtrates. Estimate the CaO in the CaC2O4 (precipitate) volumetrically by means of a standard solution of K2Mn2O8 (checked on a pure lime salt).
MgO. —The filtrate from the lime, if of large bulk, is evaporated to about 100 c.cs. in a porcelain evaporating basin, and is then transferred to a platinum dish and evaporated to dryness, and then ignited to expel all ammonia salts. Cool, add 50 c.cs. water and boil for five minutes. This dissolves out the alkalies (generally as sulphates) and any MgSO4 that may be present, and leaves as a residue MgO. Filter, wash, dry, ignite, and weigh the MgO Reserve the filtrate, which is now, after the addition of 3 or 4 drops of strong H2SO4 evaporated to dryness and ignited till constant, the weight representing MgSO4 + K2SO4 + Na2SO4. Dissolve in 50 c.cs. water. Carefully divide the solution into two equal parts. In one add a little HCl (5E.), make alkaline with NH4OH and estimate the MgO as usual with Na2HPO4.
Calculate the MgO found here and before, back to the 500 c.cs. taken (multiply by 5/3 in the first case and 5/1.5 in the second). The result is the total MgO.
K2O. Na2O.—To the second portion of the solution containing MgSO4, K2SO4, Na2SO4 add a solution of PtCl4 in slight excess. Carefully evaporate to dryness on the water bath. Take up, as before, with alcohol, and filter off the K2PtCl6, weigh and convert to K2SO4. Add the MgSO4 previously found, and subtract the K2SO4 + MgSO4 from the K2SO4 + Na2SO4 + MgSO4 previously found, and the result is Na2SO4. Convert the K2SO4 and Na2SO4 to K2O and Na2O. Take care that in these calculations account is taken of the amount of solution; that is, the fraction of the total solution employed for each estimation. Out of 500 c.cs., 300 c.cs. were taken, and this quantity was again subdivided into two lots of 150 c.cs. each. The MgSO4 K2SO4 Na2SO4 was estimated in 300 c.cs., the K2SO4 in 150 c.cs. Multiply this by 2 and subtract, and the result is MgSO4,Na2SO4 in 300 c.cs. The MgSO4 was determined in 150 c.cs. Multiply by 2 and subtract, and the result is Na2SO4 in 300 c.cs.
This multiplied by 5/3 gives the Na2SO4 in the 500 c.cs. (corresponding to 1.5 gms.) The student must in a similar manner estimate the K2SO4, and then convert the results to Na2O and K2O.
SO3.—The remaining 200 c.cs. of solution are now examined for SO3 by precipitation with barium chloride; the SO3 is calculated in the BaSO4 found, and then calculated back to the 500 c.cs., the percentage then being worked out on the 1.5 gm. taken for analysis.
CO2.—As only small quantities of this compound are, as a rule, present, it is necessary to take from 5 to 10 grams for analysis. The analysis is conducted as before described, and may be omitted at present.
The following table gives the limits within which the constituents of good “ Portland ” cements vary.
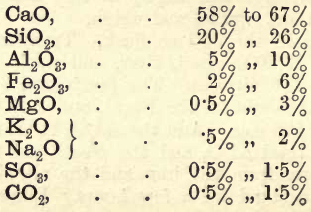
From this it will be seen that after manufacture the cement consists chiefly of silicate of lime.
Mechanical Tests
(a) Fineness.—Samples of the cement are sifted, and that which passes through a 100 mesh sieve is taken for analysis. The proportion remaining on the sieve is estimated. The finer the cement the higher its value.
(b) Resistance to Cracking. -Small cakes of cement, are made of stiff consistency, and the time of setting to withstand a wire-cutting test is noted. The cakes when dry are examined for cracks, and compared with specimens from cements known by experience to be of good quality.
Tensile Strength.— Cement, sand, and water are carefully mixed in definite proportions, and pressed into moulds under uniform conditions. The briquettes are further treated, and then tested in a suitable testing machine (the Riehle or other), and the results compared with certain standards set up for good cements.
In considering the value of a cement or fireclay, it must be remembered that the mere chemical composition is by no means the sole guiding point. In the case of a cement the degree of fineness, tensile strength, and other points also require determination ; therefore the necessary tests may be subdivided into two classes — chemical and mechanical. The purely mechanical tests lie entirely outside of the scope of this work, but on account of their importance, and to prevent the student falling into the mistake of supposing that his chemical analysis yields all the necessary information, a very brief account of the necessary mechanical tests is given.
