Table of Contents
Previous U.S. Bureau of Mines (USBM) research on the leaching of chalcopyrite with ferric sulfate [Fe2(SO4)3] demonstrates that surfactant additions enhance chalcopyrite (CuFeS2) leaching and that surfactant selection has a significant impact on the initial leaching rate. Additions of chloride ion (Cl-) are also known to enhance chalcopyrite leaching, although a review of the literature on chalcopyrite leaching reveals some controversy over the actual effect of chloride and the conditions under which it is effective.
Dutrizac and MacDonald studied chalcopyrite leaching using 0.1MFe2(SO4)3-0.1MH2SO4 (sulfuric acid). They reported a rapidly decreasing leaching rate in the absence of chloride. When 6 g/L chloride is added, leaching becomes linear. Such behavior suggests a change in leaching mechanism. They also reported that there is no leaching enhancement for chloride additions when the temperature is less than 50 °C. Munoz-Ribadeneira and Gomberg, on the other hand, reported that 0.05 to 1.0N NaCl (sodium chloride) (0.29 to 5.8 g/L) improves copper leaching at 23 to 25 °C in 1N H2SO4, while Dutrizac and others reported that 6 g/L NaCl inhibits chalcopyrite leaching in 0.1M Fe2(SO4)3-0.1M H2SO4 at 25 °C. Murr and others reported that both NaCl and KCl (potassium chloride) improve chalcopyrite leaching at pH 2.0 and 28 °C. Beane and Popp agreed that KCl enhances chalcopyrite leaching, but attributed the effect to potassium rather than to chloride. Muir and others reported that the overpotential (a measure of resistance to oxidation) developed during oxidation of sulfides in a sulfate system is not observed in chloride systems. This is attributed to chloride functioning as an electron transfer agent. Such behavior would be expected to enhance leaching in an oxidation-reduction reaction by facilitating transfer of the electron from the species being oxidized. Ruetschi and Amile reported a reversible reaction whereby copper sulfide (CuS) and Cl- convert to cuprous chloride (CuCl) and sulfur (S°). The CuS is referred to as “cupric sulfide,” but described as being lustrous blue and converts to cuprous sulfide (Cu2S) at elevated temperature in the presence of air, suggesting that it may be covellite. Such a reaction would allow chloride to destabilize any covellite formed as an intermediate during chalcopyrite leaching.
While it is hard to compare information from the various studies because of the differing conditions, there does appear to be a great deal of controversy as to the effect of chloride additions on chalcopyrite leaching. Part of the reason for this controversy could be that there is more than one phenomenon involved in the inhibition of chalcopyrite leaching. Several of the references cited above indicate that chloride assists oxidation. The previous USBM studies indicate that surfactant additions help overcome hydrophobicity of the chalcopyrite surface, thus aiding leaching. If additions of chloride overcome a chemical component (resistance to oxidation) and surfactants overcome a physical component (hydrophobic surface), a combination of the two additives might have a synergistic effect. For these reasons, a study was undertaken to more thoroughly define the effects of chloride additions on chalcopyrite leaching under chemical conditions that might be expected to exist within a dump and to determine whether leaching enhancements due to surfactant and chloride additions are coexistent.
Chloride
The most difficult task in measuring the effect of changes in solution composition on chalcopyrite leaching was finding a means of leaching at a constant ferrous-ferric ion ratio. Changes in this ratio as a result of ferric ion consumption and ferrous ion production would mask the effects of designed changes in solution composition. Such interference is especially significant when total iron concentrations are low, as would generally be the case during dump leaching. The approach chosen was to find a means to maintain a constant electrochemical potential during leaching. Since this proved to be a rather difficult but critical task, a very brief discussion of unsuccessful attempts is included to allay questions as to why a simpler experimental approach was not used.
Several methods were considered, including addition of reagents such as hydrogen peroxide, ferric sulfate, or potassium permanganate. Since there was concern that the addition of metal ions could change the leaching mechanism, initial efforts focused on peroxide additions. Preliminary tests revealed several problems in establishing either a reliable delivery system or reproducible measurements of electrochemical potential versus hydrogen electrode (Eh). Since attempts to resolve these problems were unsatisfactory, control via reagent additions was abandoned.
The other approach utilized electrochemical control by maintaining a fixed potential across two electrodes. A cell was designed employing an anionic membrane to prevent migration of metal ions to the cathode. The ferric-ferrous half cell reaction was chosen as both the anode and cathode reaction. Leaching solution was circulated in the anode compartment of the electrochemical cell to maintain the potential prior to circulation through a small column containing pure, pulverized chalcopyrite. Peroxide was periodically added to the catholyte to regenerate the ferric ion necessary for the cathode reaction. Problems in balancing solution flow caused the leaching column to be abandoned.
The best results were obtained by adding ground chalcopyrite directly to the anode compartment of the cell. With this approach, it was possible to perform tests at constant electrochemical potentials regardless of the total iron concentration and to statistically evaluate the effect of additives.
Materials and Procedures
Leaching tests were done in the membrane cell developed during preliminary studies (figure 1). A personal computer and data acquisition system was used to simultaneously operate eight cells at different potentials. Input to the computer was a signal from a double-junction dual-purpose Pt/Ag/AgCl electrochemical probe (figure 1A) inserted through the lid of each cell. The probe was positioned away from the electrodes to monitor the average electrochemical potential within the compartment. The control system was programmed to apply power to the electrodes whenever the signal from the probe fell below the designed set point. When constant-potential tests were run at low oxidation potential, air entering the leaching cell oxidized the iron in solution, increasing the solution potential beyond the set point, a situation the control system was not designed to handle. Argon bubbled into the solution provided an inert blanket, preventing air from entering, avoiding the problem of exceeding the set potential. Glass tubing (figure 18) connected to a constant temperature bath was used as a heat exchanger to maintain constant temperature in the leaching solution.
The cell utilized two sheets of anionic membrane, separated by a plastic grid (figure 1, C and D), to minimize copper transport. Iridium-coated titanium electrodes (figure 1E) were used to minimize any reaction involving the electrodes. Electrodes were fixed in place utilizing slots attached to the cell walls. Each electrode was 0.6 cm from the membrane.
The experimental design utilized in this testing was a 2³ factorial design. Three 2³ factorials were conducted for 8 days, one each at 630, 700, and 750 m V Eh, in the constant-potential leaching cells. The ferric ion proportions to which these Eh values correspond are shown in table 1. The values were determined by mixing the desired proportions of reagent -grade ferric and ferrous sulfate, adjusting the pH to 1.7 using reagent-grade sulfuric acid, and measuring the Eh at 50°C. The electrode used for Eh measurements was standardized using a commercial Eh standard of 675 m V Eh.
The experimental conditions for the factorial designs are displayed in table 2. Conditions for iron and surfactant concentrations were chosen to cover the ranges previously studied. Chloride concentrations were

based on two criteria: (1) concentration low enough to minimize concerns about corrosion and (2) preliminary tests, which indicated rapidly decreasing leaching enhancement from additions greater than 2 g/L. Iron and chloride additions were made with reagent-grade ferrous sulfate and NaCl. With the exception that no sodium chloride was added to the catholyte, solution compositions in both chambers of the cells were identical at the start of each test. The constant-potential cells were used to adjust the Eh to the desired level for each test. Each design was conducted at 50 °C, pH 1.7, and 0.5 pct CuFeS2 in 1 L of solution. The chalcopyrite sample utilized was specimen-grade mineral from Transvaal, Republic of South Africa. Quartz was found to be the only impurity, and contamination was minimized by hand sorting. The coarse mineral specimens were crushed, ground, sized, and stored under an inert gas to minimize surface oxidation. The size fraction used in all tests was minus 200 plus 400 mesh. The nonionic surfactant tested was LA 790, a polyoxyethylene lauryl ether, which was found to be the superior surfactant in previous leaching tests.
Results and Discussion
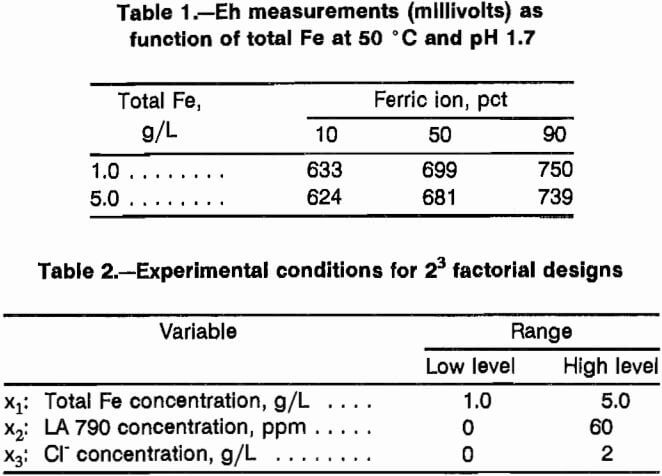
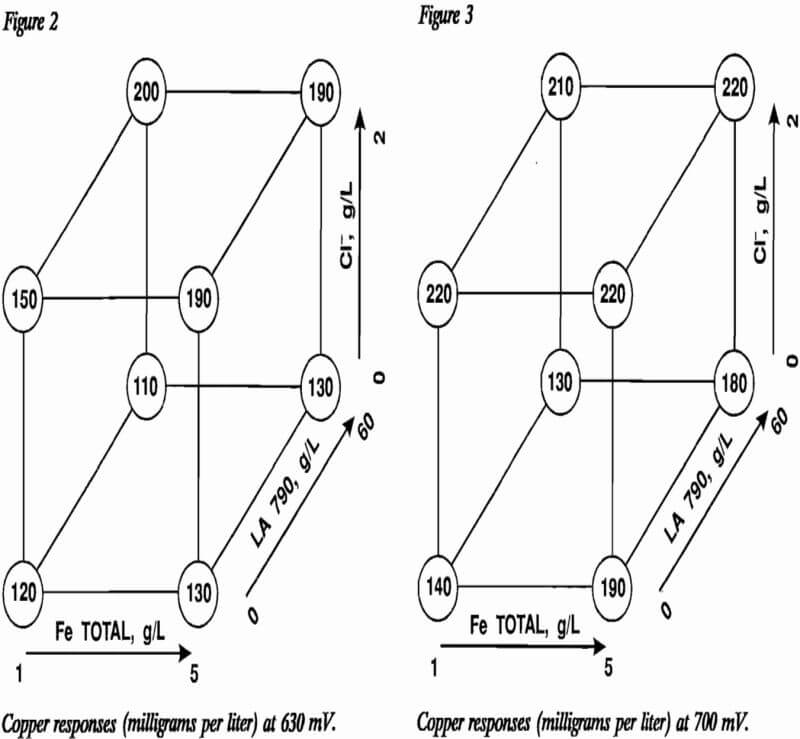
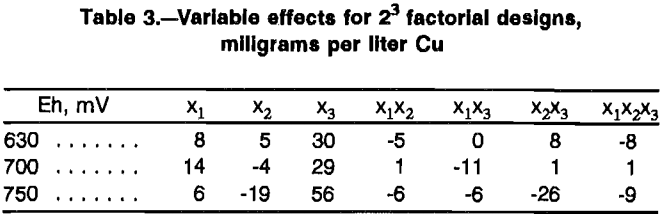
The results of the three factorial designs are displayed in figures 2 through 4. The variable effects as calculated from the factorial designs are presented in table 3. The effects that are statistically significant in light of experimental error can be determined using the t-distribution. Since an estimate of experimental error is required, an estimate of 23.18 mg/L Cu with eight degrees of freedom was determined from replicate experiments conducted during the shakedown rims of the constant-potential leaching cells. The significance test is conducted using the equation:
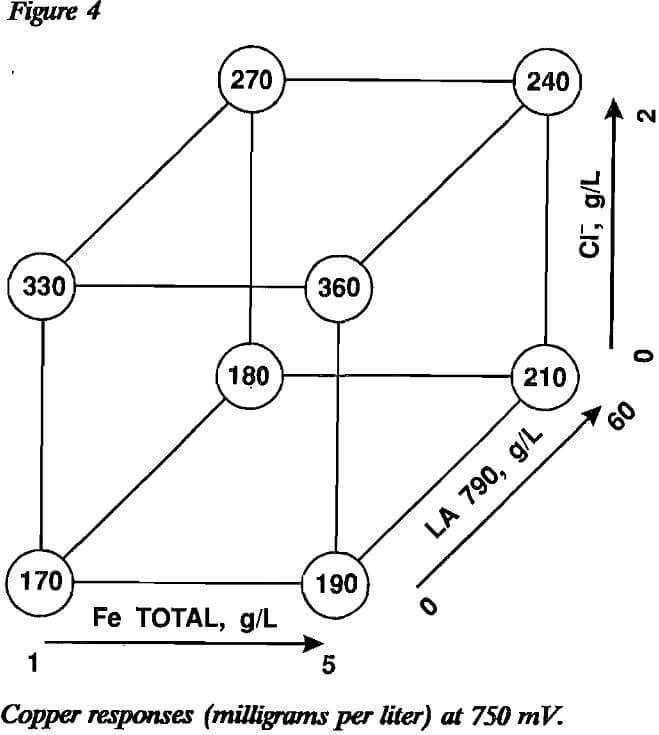
90-pct confidence interval = effect ± ts(4/8)½
where t = value from t-distribution for 90 pct confidence (two-tailed),
and s = estimate of experimental error.
The slightly liberal 90-pct confidence interval was selected because the factorial designs were not replicated to obtain a more precise estimate of experimental error as part of the testing. Using this formula, the 90-pct confidence interval was determined to be
90-pct confidence interval = effect ± 30 mg/L Cu.
An effect is judged to be significant or real only when the absolute value of that effect is greater than the confidence interval.
When applied to the individual effects of table 3, only one effect, the effect of Cl- at 750 mV Eh, is significant.
However, the Cl- effects at 630 and 700 mV Eh are substantially larger than the other effects, suggesting that the employed estimate of error may be too large. The estimate can be improved by incorporating additional measurements of random error. One way to accomplish this is to assume that interactions exhibiting small measured effects produce an actual effect of zero. The three-factor interaction is often chosen in such cases since three-factor interactions are rare. The three values from this column can then be assumed to be random error and can be pooled with the previously used values to calculate a new estimate of error. After doing this, the estimate of error became 20.10 mg/L Cu with 11 degrees of freedom and the 90-pct confidence interval became ±26. With this estimate of error, the Cl- effects at 630 and 700 mV Eh are significant with 90 pet confidence.
Further evidence that the effects of chloride additions are real comes from comparison of results between the planes representing 0 and 2 g/L Cl- in figures 2 through 4. Ratios of any two results differing only by the presence or absence of chloride demonstrate that copper leaching enhancements of 14 to 94 pct can be achieved. Thus, chloride additions improved the leaching rate of chalcopyrite under the conditions studied. Nonionic surfactant addition, as well as any interactions associated with nonionic surfactant addition, had no significant effect on leaching. The effect of the interaction between nonionic surfactant concentration and Cl- concentration at 750 mV Eh is borderline negative. The effect of total iron was not found to be significant within the range of 1 to 5 g/L.
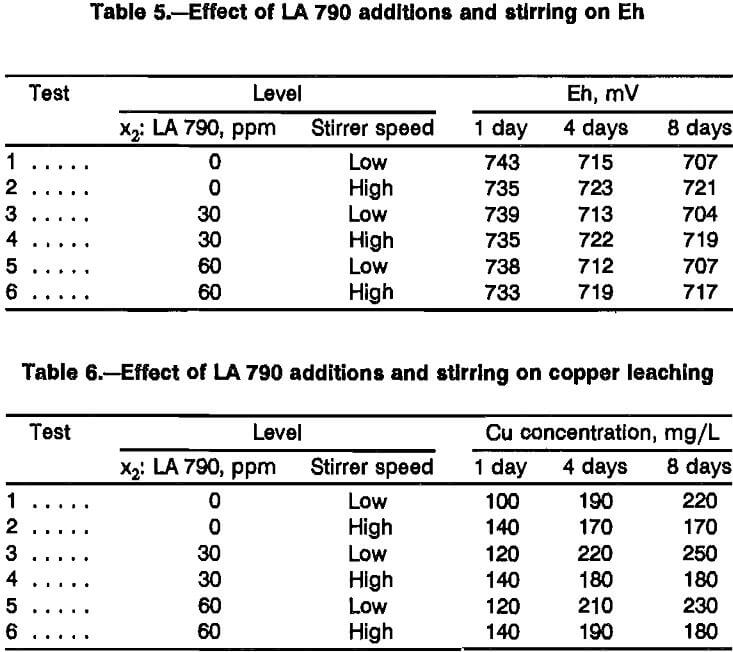
The conclusion that surfactant contributes no significant effect was surprising considering the results of previous work. The main difference between these leaching tests and the shake flask tests conducted previously was the degree of mixing (i.e., a high degree of mixing in the constant-potential leaching cells versus a low degree of mixing in the shaker flasks). This suggested the possibility that the effect of nonionic surfactant additions on leaching rate interacts with the degree of mixing. To test this premise, a set of six tests was done in which both surfactant addition level and stirrer speed were varied using the variable levels defined in table 4. High stirring corresponds to the level used throughout the previous testing with chalcopyrite suspended in the leaching solution. Low stirring corresponds to gently agitating the leaching solution without suspending the chalcopyrite, similar to the conditions that existed during shake flask tests. The fixed variables were the same as those used in the previous tests plus 3 g/L Fe as ferric sulfate. Although the constant- potential cells were used, voltage was not applied to avoid the possibility of polarization of chalcopyrite surfaces, more closely representing the conditions in the shake flask tests. The Eh was measured throughout the course of the tests (table 5). While it was observed to decrease, the Eh was never less than 700 mV. By comparison with the data in table 1, leaching solutions were never predominantly ferrous and cessation of leaching would not be a result of depletion of reactant.
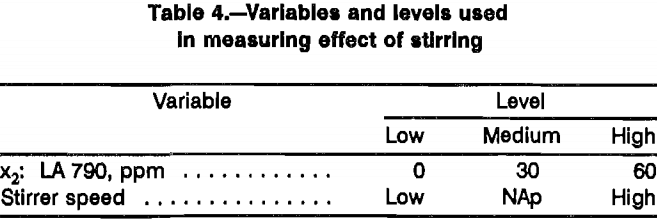
While there is no clear evidence for any effect by surfactant and more tests would have to be run to obtain statistically meaningful results, the results of these tests (table 6) suggest complex interactions involving electrochemical potential, surfactant addition, and degree of mixing. Definition of these interactions would require a detailed study and could not be justified as part of this investigation since no enhancement for surfactant additions could be verified. Conversely, leaching enhancement from surfactants interaction cannot be ruled out since different responses were measured between stirred cells and unstirred cells. This demonstrates that stirred cells may be invalid models for the effect of surfactants during dump leaching.
Conclusions
In the constant-potential leaching cells, addition of Cl- to ferric sulfate for leaching of chalcopyrite at 50 °C, pH 1.7, and 1 to 5 g/L total Fe improved the leaching rate at 630, 700, and 750 mV Eh. Thus, under the chemical conditions encountered within a dump, levels of chloride up to 2 g/L will enhance leaching of chalcopyrite by as much as 94 pct. The presence of chloride will also increase corrosion, however, and would have to be evaluated on a site- by-site basis to determine its utility.
The effect of surfactant additions was less clear. While previous research demonstrated that addition of the nonionic surfactant LA 790 had a positive effect on chalcopyrite leaching in gently mixed shake flask tests, no effect was demonstrated in the stirred cells. With gentle stirring, similar to the mixing achieved in shake flasks, the influence of surfactant addition was consistently positive, but the effect was too small to ensure that it was statistically significant. There was no verifiable interaction between surfactant and chloride additions.
