The direct conversion of mineral concentrates into useful ceramic materials, without several intermediate processes, is of great interest to the materials community. Recently there has been increased interest in producing ceramic powders with very small sizes (in the order of nanometers) starting with mineral precursor. Thermal plasma processing of mineral concentrates may be used to obtain ceramic powders useful for advance material applications without many of the environmental difficulties that alternative processes may have.
For many years, ilmenite has been a major source of titania (TiO2) for pigments, and more recently, as a source of titanium and iron. TiO2 is used as a pigment in paint, paper, rubber, ceramics, plastics, textiles, linoleum, printing ink, cosmetics, and soap. Many processes have been used in treating ilmenite to obtain a variety of products. The direct reduction of ilmenite with carbon in an electric furnace leads to the production of liquid iron and a TiO2 rich slag. This slag has been recovered and used directly as a pigment, or reduced even more to obtain metallic titanium. Other processes studied by Brown, include reduction of ilmenite with hydrogen, methane or ammonia in a plasma jet reactor, where the principal products are TiO2 and iron. Metal- matrix composites are the subject of substantial research and development all around the world, specifically in developing particulate Fe based metal-matrix to produce tools with high toughness and wear resistance. One such type of composite is the TiC-Fe system, where small particles of TiC are finely dispersed in the Fe matrix.
Another mineral concentrate that has been converted into desirable products using plasma technology is zircon (ZrSiO4). Zircon is one of the most common and widely used zirconium minerals. It is generally found in river and beach sands along with other minerals such as ilmenite, rutile, monazite and garnet. Given its abundance and low cost it is the preferential starting compound for zirconium based materials, such as zirconia (ZrO2) and zirconium carbide (ZrC). Zirconia has been produced by the fusion of zircon sand with sodium carbonate or caustic soda, and by the high temperature reaction of zircon with calcium oxide, magnesium oxide or soda ash by. Zirconium oxide can be produced directly from zircon by thermal decomposition in an electric furnace or a plasma reactor. The product of both processes is dissociated zircon that consists in a mixture of zirconia (ZrO2) and silica (SiO2). The silica is subsequently leached out by boiling caustic soda or sodium hydroxide. The microstructure of the plasma dissociated zircon (PDZ) has been studied indicating that the zirconia crystallites appear in a silica matrix following spherulitic growth. Zirconia has been used as an important base ceramic for different composites and as an additive to pigments.
Experimental Procedure
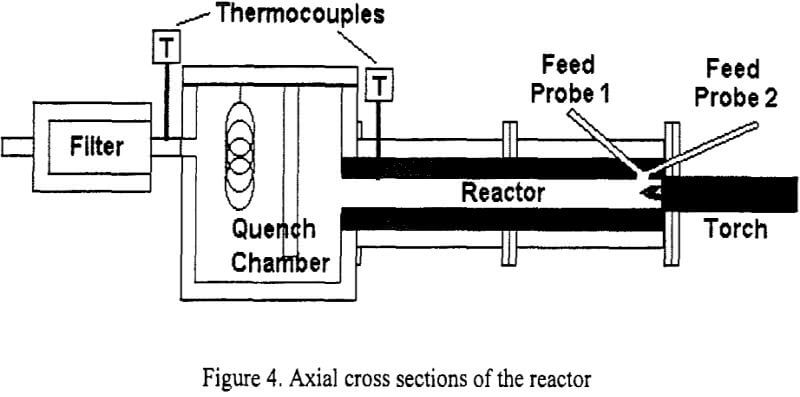
The reactor used for the experiments was designed and built by Taylor and Pirzada, for the synthesis of ultra-fine silicon carbide particles. The system consists of a PEC PT-50 plasma torch, a tubular flow-type reactor, quenching chamber and filter, powder feeder, data acquisition system and gas circuit. Figure 4 shows the axial cross section of the reactor where the main parts are indicated. A complete description of the experimental system can be found in the work done.
For the TiC-Fe synthesis, grounded -270 mesh ilmenite concentrate from RGC Mineral was used. Characterization using x-ray diffraction showed that the mineral consisted of ilmenite and rutile (TiO2) as main constituents. Table 1 shows the chemical composition of the concentrate.
Two different types of zircon concentrates were used for the thermal decomposition into zirconia and silica, and for the synthesis of zirconium carbide. These concentrates from Australia and Venezuela present a chemical composition described in Table 2.
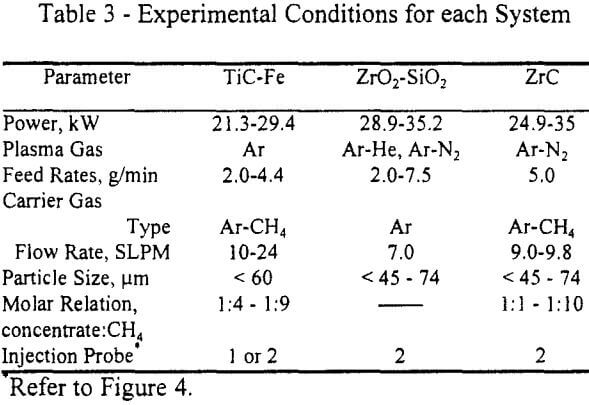
The operation of the system was practically the same for all the experiments. The plasma torch was started using argon as plasma gas, and after the plasma was stable either nitrogen or helium (depending on the experiment) was mixed with the argon. Once a thermal steady state was achieved in the system, the concentrate was fed though the feeding probe using argon or argon-methane as carrier gas for at least 15 minutes. Powders were collected in the reactor, quench box and filter. Table 3 shows the experimental conditions used in each case.
The products obtained in each experiment were characterized by x-ray diffraction, SEM, TEM and wet chemical analysis. A complete description of the characterization can be found elsewhere.
Powders of TiC-Fe, ZrO2-SiO2 and ZrC-SiC were successfully produced from ilmenite concentrate and methane, zircon, and zircon plus methane respectively, using a non-transferred arc thermal plasma reactor. Maximum conversion of 97% for ilmenite to TiC was obtained using 25 kW of input power. The maximum conversion from zircon to zirconia and silica was 94%, while the conversion to zirconium carbide using methane was 60%. The size of the powders obtained were in the sub-micron range. In general, increasing the input power increases the conversion of the concentrates; increasing the initial size of the concentrates decreases the conversion; an increase in the amount of methane in the ilmenite system increases the formation of TiC, and decreases the ZrC formation in the zircon-methane system.

