Table of Contents
Extensive literature, published during recent years, identifies three general methods of fine-particle preparation: (1) solution techniques, (2) vapor-phase techniques, and (3) salt decomposition techniques. Solution techniques, also referred to as “chemical” preparation, offer the advantages of ease of preparation, good control of composition, and homogeneity. Among the solution techniques a number of terms, such as “codecomposition” “evaporative decomposition from solutions” (EDS), “citrate process”, and “alkoxide process” are frequently encountered.
Vapor-phase techniques are receiving more and more attention, due mostly to interest in high-performance structural ceramics such as SiC and Si3N4. These techniques lead to unaggregated powders. Among the techniques in this category are all plasma methods and others involving gaseous reactants.
Decomposition of precursor salts (e.g., oxalates) has been employed in the preparation of fine-powder carbides.
BN powders have been prepared by the reaction of boron oxides and borates with ammonium chloride (NH4Cl) in N2 and NH3 atmospheres. In certain reactions, in order to increase the reaction rates in the solid state, that is, to effectively increase the reaction surface areas, inert fillers such as CaCO3 and calcium phosphates have also been employed. Ammonium salts other than NH4Cl have also been used in BN synthesis.
Ammonium thiocyanate, for example, resulted in much higher reaction rates with boric acid in the BN synthesis. Among the less conventional laboratory techniques reported for the preparation of nonoxide ceramic powders are the reaction of compounds (such as silanes, boranes, and halides) containing the desired cations in super-high-frequency discharge plasma, chemical vapor deposition techniques, and the use of laser energy. Similar reactions have also been studied employing conventional high-temperature furnaces at 1,500° to 1,900° C.
Production of complex nitride powder has been studied by the nitridation of metal halides (SiCl4, BCl3) in an N plasma. Chemical vapor deposition techniques have also been used to prepare thin films of BN, Si3N4, and TiN. Extremely fine, uniform Si3N4 and SiC powders have been synthesized from SiH4, NH3, and C2H4 gas-phase reactants heated by a CO2 laser. Nitrides of Si, B, and Ti have also been prepared by the reaction of their halides with ammonia or ammonia salts at high temperatures.
No mention of preparing BN from halides of B with the reaction of ammonia salts or ammonia in the liquid phase has been made in the literature. In view of this finding, research efforts included a study of obtaining ultrafine BN powders from the reactions of halides with NH4Cl and NH3 in the liquid phase.
Experimental Equipment and Procedures
Reactions Between 500° and 750° C
Two different approaches were evaluated for the synthesis of fine BN powders. The first was the reaction between an inorganic B compound and an organic N compound between 500° and 750° C in an NH3 atmosphere. For this a high-temperature controlled-atmosphere furnace was used. Inorganic B compounds used included boric oxide (B2O3), boric acid (H3BO3), borax (Na2B4O7-10H2O) , and anhydrous sodium tetraborate (Na2B4O7). Carbimide (NH2·CO·NH2) and thiocarbimide (NH2·SO·NH2) were used as N2 sources. The powdered reactants were mixed in stoichiometric proportions and placed in the furnace. The furnace chamber was evacuated and purged with NH3 gas before the temperature was raised. Experiments ran from 4 to 24 h at 500° to 750° C.
Reactions Between -75° and 200° C
The second approach involved the synthesis of BN through reactions between various N and B compounds leading to the formation of possible elemento-organic compounds as intermediate precursor species, the decomposition of which might lead to BN formation. Such compounds included NH3, boron halides (principally boron trichloride, BCl3), ammonium chloride (NH4Cl), and sodium borohydride (NaBH4).
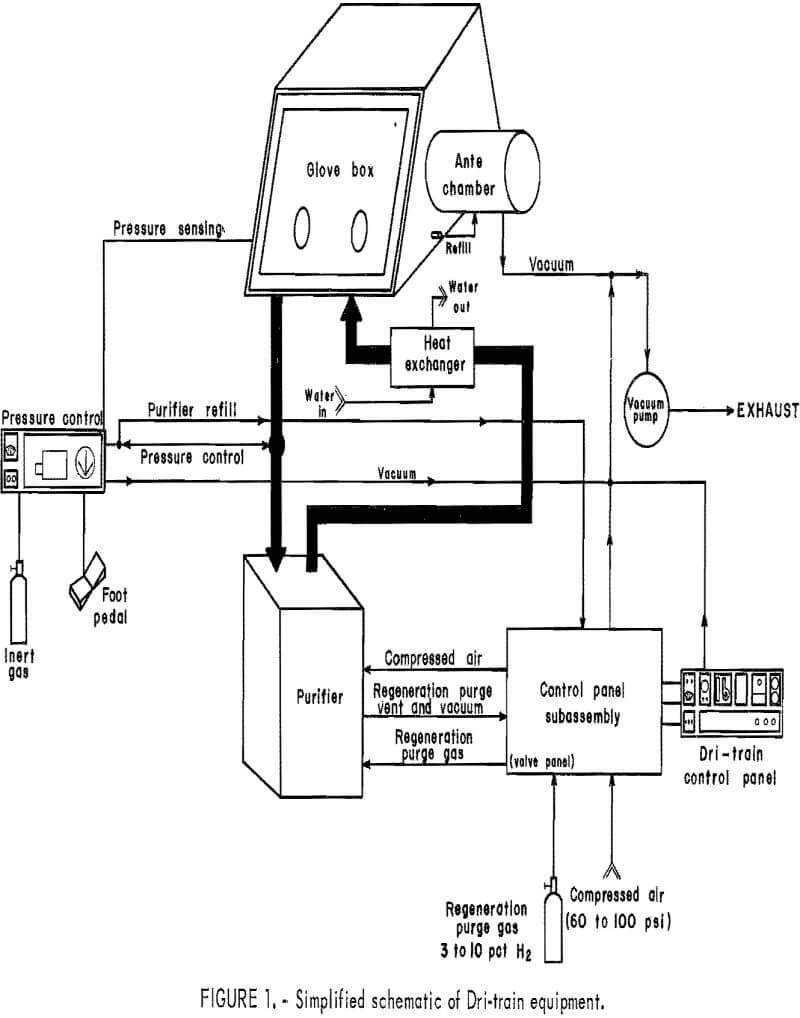
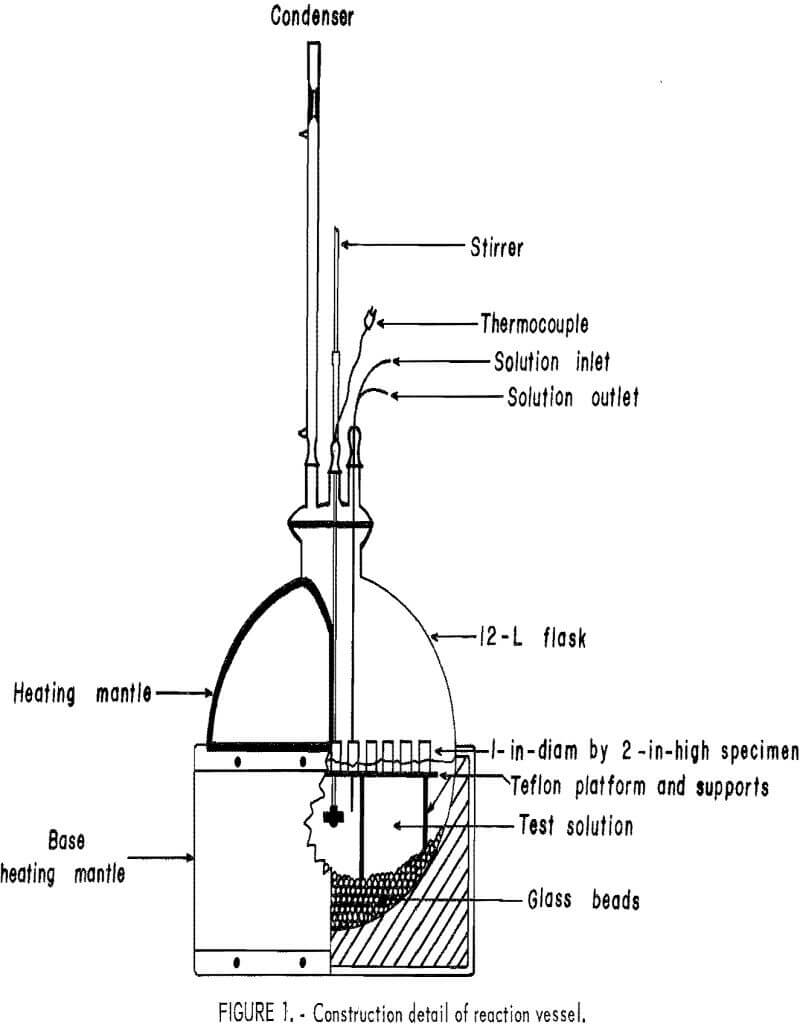
For the second approach, material preparation and handling procedures were performed in a dry-box system, a simplified schematic of which is shown in figure 1. The atmosphere in the box was maintained by a VAC Dri-train unit capable of generating an inert gas atmosphere containing less than 1 ppm O2 and/or moisture. This high-purity inert atmosphere was obtained by circulating the gas in a closed system going through a purifying chamber equipped with molecular sieves to trap O2 and moisture. Figure 2 shows the dry-box assembly. Reactions were carried out in the experimental unit (fig. 3) consisting of a three-necked, 500-mL glass reaction vessel and a small vacuum system with cold traps.
Three systems were studied. They included ammonia-boron halide, ammonium salt-boron halide, and ammonium salt- metal borohydride reactions.
Results and Discussion
Acidproof Materials
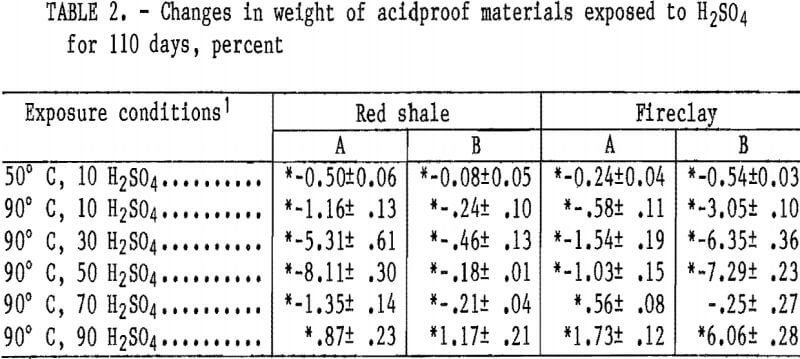
Statistically significant weight changes occurring in the acidproof materials are listed in table 2. All materials showed an increase in weight loss in 10 wt pct H2SO4 when the temperature was increased from 50° to 90° C. At 90° C, the materials showed an increase in weight loss with an increase in acid concentration up to either 30 or 50 wt pct H2SO4, and then a decrease in weight losses at 70 wt pct. At 90 wt pct H2SO4, all samples showed a weight gain. Red shale A and fireclay B samples, which have the highest apparent porosity of the acidproof materials, have the highest weight losses, of 8.11 and 7.29 wt pct, respectively, when exposed to 50 wt pet H2SO4 at 90° C. The largest weight losses for the low-porosity red shale B (0.46 pct) and fireclay A (1.54 pct) materials occurred in 30 wt pct H2SO4 at 90° C.
Cold crushing strength data for the acidproof materials are listed in table 3. No discernible trends were observed for any of the red shale or fireclay samples and no significant differences exist between cold crushing strength values of the top (vapor exposed) and the bottom (liquid exposed) halves of the test samples.
Note.—Plus-minus (±) values are at 95-pct confidence intervals.

Note.—Plus-minus (±) values are at 95-pct confidence intervals.
The weight percent of ions leached from the acidproof materials are listed in table 4. Silicon was leached from samples in 10-pct-H2SO4 concentrations and, except for one instance, was not removed at higher acid concentrations. Calcium was removed in trace amounts (less than 0.033 pct) in all acid concentrations. As temperature or acid concentration was increased, less calcium was removed. All other ions exhibited an increase in quantity leached from the samples with an increase in temperature at the same acid concentration. As acid concentration was increased from 10 to 90 pct at 90° C, the quantity of ions removed from a material increased to a maximum value at either 30 or 50 wt pct H2SO4, then decreased with atmosphere with reaction times of 24 h or longer, formation of BN was observed. This reaction, however, was low yielding, probably owing to the decreased reactive surface area of the boric anhydride upon melting at 450° C. To increase the yield, the effective surface area of the boric acid was increased by the addition of tricalcium phosphate (Ca3(PO4)2) as an inert filler. This resulted in BN yields as high as 75 pct for reaction times of 24 h. The synthesis process may be represented by the following reaction:
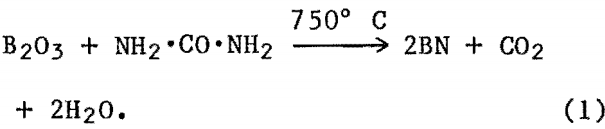
When borax and carbimide were used as reactants in an NH3 atmosphere, BN formation proceeded much faster than in the boric acid-carbimlde system. The reaction could be represented by—
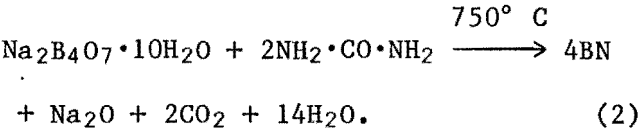
Reaction products obtained were washed in dilute HCl, and the insoluble portion was filtered out. X-ray diffraction (XRD) analysis of the filtered powder indicated that it was amorphous. Heat treatment of this material at 1,400° C in an N2 atmosphere for several hours resulted in crystalline BN powder based on XRD patterns. The BN yield of reaction 2 was as high as 95 pct of expected theoretical value. It appears that dehydration of borax at temperatures (200° to 400° C) below the reaction temperature results in a sponge-like anhydrous borax with a very high surface area. This, along with the decomposition of the carbimide to produce additional NH3 gas of high reactivity, results in higher BN yields.
The same reaction run in an N2 atmosphere resulted in lower yields. The higher yields produced in an NH3 atmosphere compared to an N2 atmosphere may be due to the higher reactivity of N2 produced by NH3 decomposition at 450° C.
The H2 produced as a result of NH3 decomposition might also have a catalytic effect on the reaction, resulting in higher yields.
The use of thiocarbimide (NH2SONH2) as the organic N2 source further increased the reaction rate as, evidenced by the shorter time required for the reaction to reach completion. In the reaction with thiocarbimide, sodium sulfides were produced as potential reaction byproducts, which may further decompose at about the reaction temperature of 750° C. This decomposition process at the BN-formation temperature results in spongelike high- surface-area material, thus accelerating the reaction rates.
Reactions Between -75° and 200° C
Ammonia-Boron Halide Reactions
Once the reactants (NH3 and BCl3) are introduced into the three-neck reaction flask, reaction starts immediately, as evidenced by formation of a white powder around the walls of the reaction flask. This reaction is allowed to continue for 2 to 3 h. At the end of such time the excess liquid NH3 remaining in the flask is removed by vacuum, the white powdered reaction product is isolated and dried at room temperature. The following two reactions appear to take place either concurrently or consecutively:
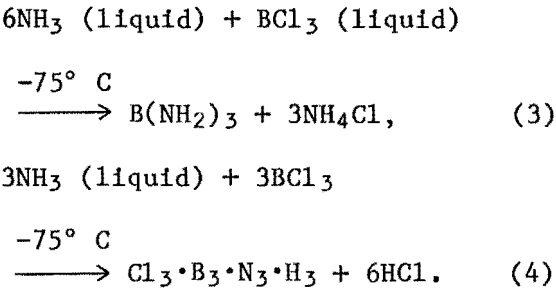
The intermediate reaction products, elemento-organic BN compounds, that may be formed in these reactions have not been isolated and identified. The general chemistry of these systems, however, suggests the formation of such compounds and the precipitation of BN as a result of their carbothermic decomposition.
An attractive feature of these re-actions appears to be their general applicability to the preparation of other nitrides, such as AlN, TiN, and Si3N4. Future efforts should be directed toward a study of the reaction mechanisms through the isolation and characterization of the intermediate precursor com-pounds. Understanding these reactions would help determine the feasibility of the formation of other nitride compounds at low temperatures.
XRD analysis of the dried material identified the presence of NH4Cl in reaction 3; detection of HCl fumes through the mercury bubbler in the reaction set confirmed reaction 4.
The rest of the powder in the reaction products was amorphous to X-rays. After being heated in vacuum at 200° to 250° C for several hours, the powder was still amorphous to XRD. Heat treatment of the powder at 1,200° to 1,400° C for several hours resulted in BN detectable by XRD. A chemical analysis of the powder after washing in dilute HCl acid solution and filtration revealed 42.4 pct B, 53.1 pct N, and 0.7 pct 0. Low O2 content of the sample illustrates the appropriateness of the method for preparing high-chemical-purity BN compared to borax technique (O2 = 5 pct) . Surface area of BN powder prepared by this method was measured to be 80 to 100 m²/g depending on the temperature of crystallization, Surface area and particle size of a commercially available BN powder were determined to be 10 m²/g and 45 µm, respectively. Densities (helium pycnometer) were 2.18 to 2.21 g/cm³.
Ammonium Salt-Boron Halide Reactions
As previously described the reaction was allowed to proceed for 8 to 12 h. The flask was then cooled to room temperature, and the organic solvent was removed by vacuum followed by heating the solid residue in vacuum to 200° to 250° C for several hours. XRD analysis of reaction product(s) showed an amorphous component and NH4Cl. Further heat treatment of this powder at 1,200° to 1,400° C in vacuum or N2 atmosphere resulted in BN powder detectable by XRD. A possible reaction sequence may be—
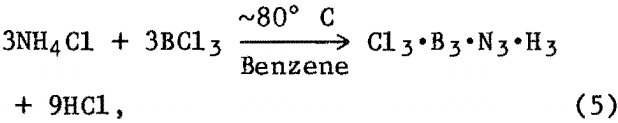
then, upon heating in vacuum at approximately 250° C,
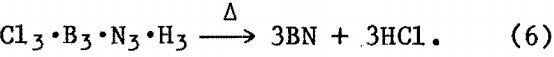
Elemental analysis of the BN thus obtained gave 42.8 pct B, 54.1 pct N, and 2.5 pct O, which is similar to the composition of the BN powder from the ammonia-boron halide reaction and compares favorably with theoretical composition of BN (B/N = 0.79).
Surface area and density measurements gave values ranging from 80 to 110 m²/g and 2.15 to 2.20 g/cm³, respectively.
Ammonium Salt-Metal Borohydride Reactions
White powder formed by the NH4Cl + NaBH4 reaction was isolated upon the removal of organic solvent by vacuum and heating of the sample to 200° to 250° C under vacuum for several hours. As with the two previous reaction systems, this powder was amorphous to X-rays; only upon heat treatment at 1,200° to 1,400° C in vacuum or N2 for several hours was BN detected by XRD analysis.
Chemical analysis of the powder showed 42.0 pct B, 52.7 pct N, and 2.3 pct O, which again compared well with the theoretical BN composition and the powder from the other two reaction systems described.
Values of 70 to 200 m²/g and 2.15 to 2.22 g/cm³ were obtained for the surface area and density, respectively.
In addition to surface area, density and elemental analysis attempts were made to measure the particle size by direct methods; namely, by scanning electron microscope (SEM) and X-ray line- broadening techniques.
Note.—Plus-minus (±) values are at 95-pct confidence intervals.
Note.—Plus-minus (±) values are at 95-pct confidence intervals.
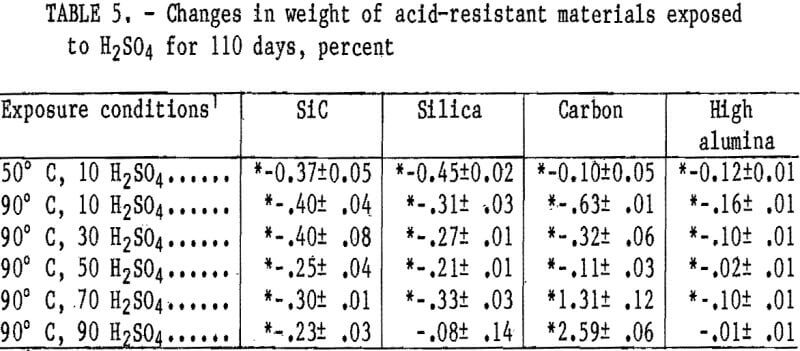
and acid concentrations, but showed a weight increase at 90° C in 70 and 90 wt pct H2SO4, reaching a maximum value of 2.6 wt pct in 90 wt pct H2SO4. The reason for these weight increases has not been determined.
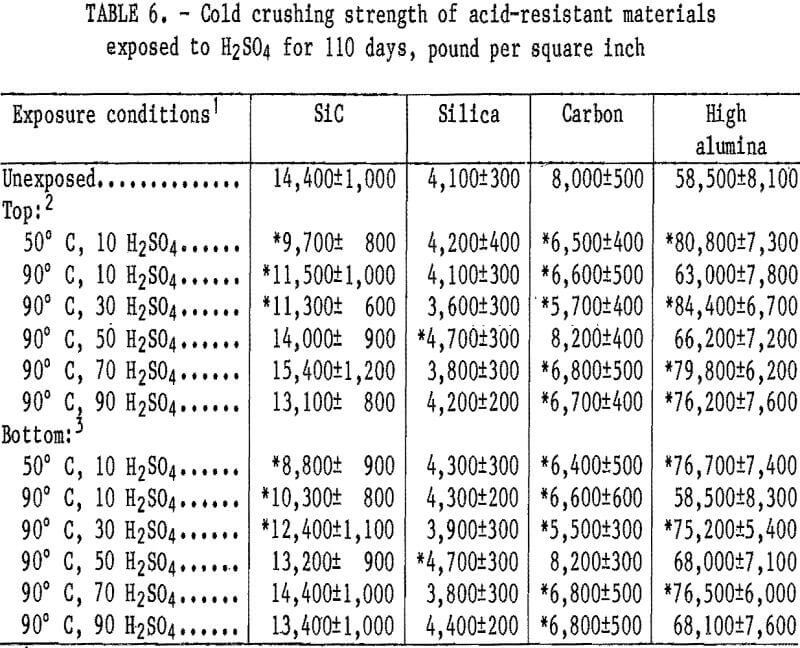
Cold crushing strength values are listed in table 6 for the acid-resistant materials. A statistically significant decrease in crushing strength from 8,000 to about 6,500 psi occurred in the carbon material at all acid concentrations and temperatures, except at 50 wt pct H2SO4 and 90° C. The SiC material had a statistically significant decrease in crushing strength from 14,400 to about 11,000 psi that occurred in 10 wt pet H2SO4 at 50° C and 10 and 30 wt pct H2SO4 at 90° C. The high-alumina and silica samples show no statistically significant strength losses.
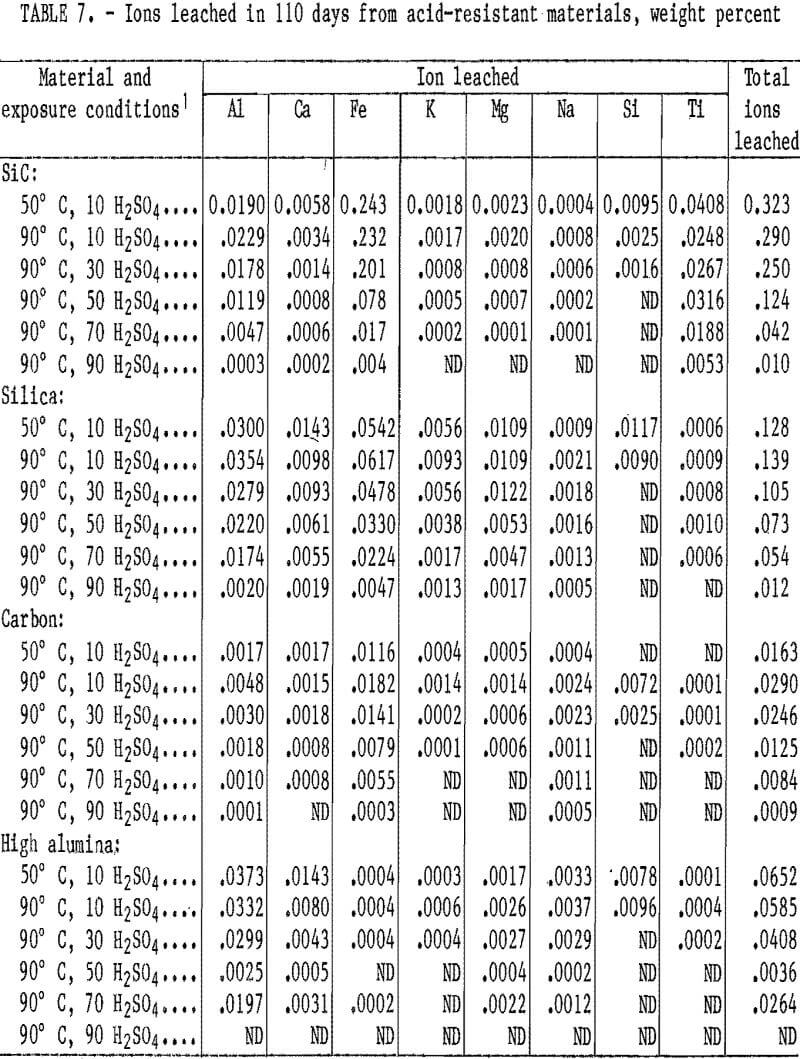
The weight percentages of ions removed from the acid-resistant materials exposed to H2SO4 for 110 days are listed in table 7. During the 110-day leach, the ion removal rate from the four acid-resistant materials tended also to fit the second- order parabolic equation described in the “Acidproof Materials” section, with 94 out of 152 curves yielding coefficients of correlation above 0.95. The quantity of ions removed from samples generally decreased when acid concentration was increased from 10 to 90 wt pct H2SO4 at 90° C. The quantity of ions removed from samples exposed to 10 wt pct H2SO4 did not show a trend when temperature was increased from 50° to 90° C. In all acid- resistant materials, either the Al or Fe ions were leached in the largest quantities. The greatest amount of a single ion removed (0.24 pct Fe) occurred in the SiC material at 50° C and 10 wt pct H2SO4. All other ions were leached in quantities less than 0.07 pct.
The total ion leach data for the acid-resistant materials are shown in figure 3. All samples shown have a decrease in the total ions removed when acid concentration was increased from 10 to 90 pct at 90° C, with no ions removed in 90 pct H2SO4. The SiC and the high-alumina samples show a decrease in the total quantity of ions leached in 10 wt pct H2SO4
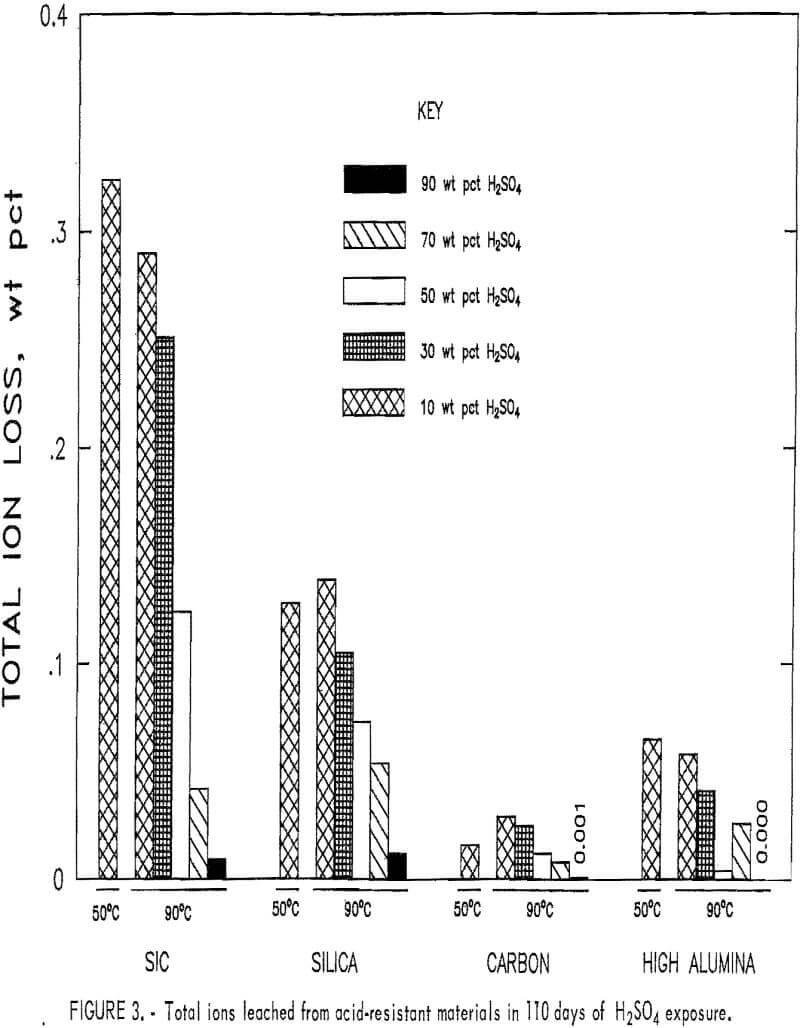
when temperature was increased from 50° to 90° C, and the silica and the carbon showed an increase. The largest amount of total ions leached, 0.32 pct, occurred in the SiC material exposed to 10 wt pct H2SO4 at 50° C. The carbon and high-alumina samples had the least total ions leached. Less than 0.03 wt pct of total ions was leached from the carbon and less than 0.07 wt pct from the high-alumina samples under the conditions studied.
Scanning electron microscopy, electron probe, and XRD examinations of the acid-resistant materials indicated no phase changes occurred. No correlation existed between ion leaching and cold crushing strength values. The carbon material, which had nearly a continual strength decrease under the conditions investigated, had the lowest total ion removal of any material tested.
Conclusions
Samples of two red shale, two fireclay, one SiC, one silica, one carbon, and one high-alumina ceramic materials were exposed to 10 wt pct H2SO4 for 110 days at 50° C, and to 10, 30, 50, 70, and 90 wt pct H2S04 for 110 days at 90° C. A study of the corrosion effects after this 110-day period of exposure to H2SO4 produced the following indications;
- The silica and the high-alumina samples had the best acid-resistance properties.
- Total ions leached and sample weight loss values were generally highest at exposures of 30 to 50 wt pct H2SO4 at 90° C for the acidproof materials and at 10 wt pct H2SO4 for the acid-resistant materials.
- The highest density red shale and fireclay materials had good acid-resistance properties, with a maximum total ion weight loss of 0.90 and 1.06 wt pct, respectively. The total ion weight loss of the acidproof material was directly related to initial apparent porosity.
- In general, the Fe and Al ions had the highest removal rates, while removal of Ca, Mg, Na, K, and Ti ions removal was minor.
