Table of Contents
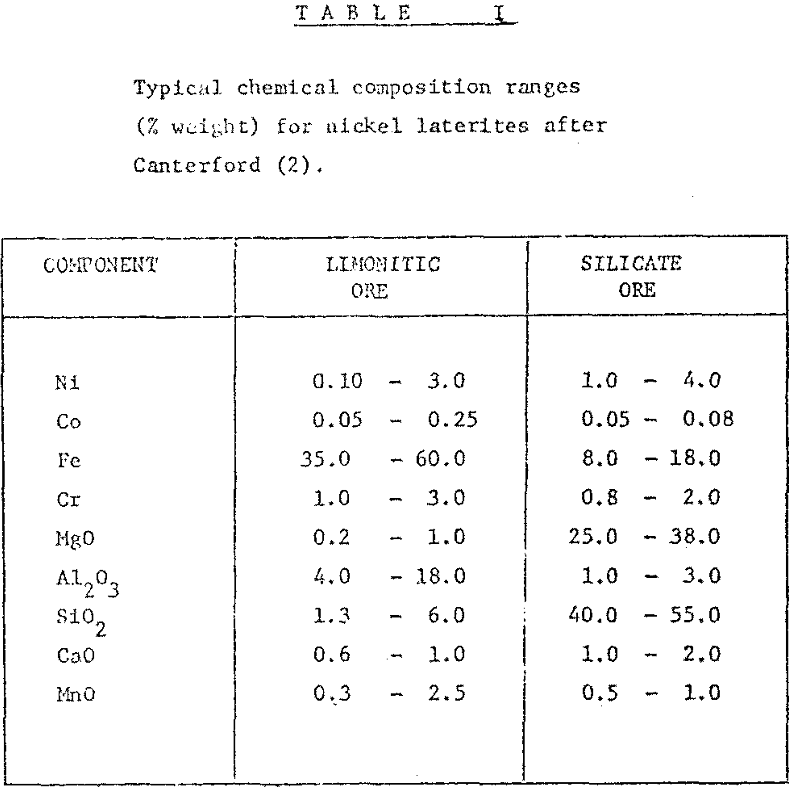
Nickel ores may be classified into two major categories : sulphide ores, and oxidized lateritic ores, these latter containing nearly 80% of the known reserves of the metal. Unfortunately, no economically viable process for the concentration of nickel from low-grade deposits has yet been developed, and so only the high grade ores are able to be worked profitably. Silicate ores have been treated pyrometallurgically for the production of ferronickel; limonitic ores have been processed hydrometallurgically to produce nickel metal.
The Cementation Step
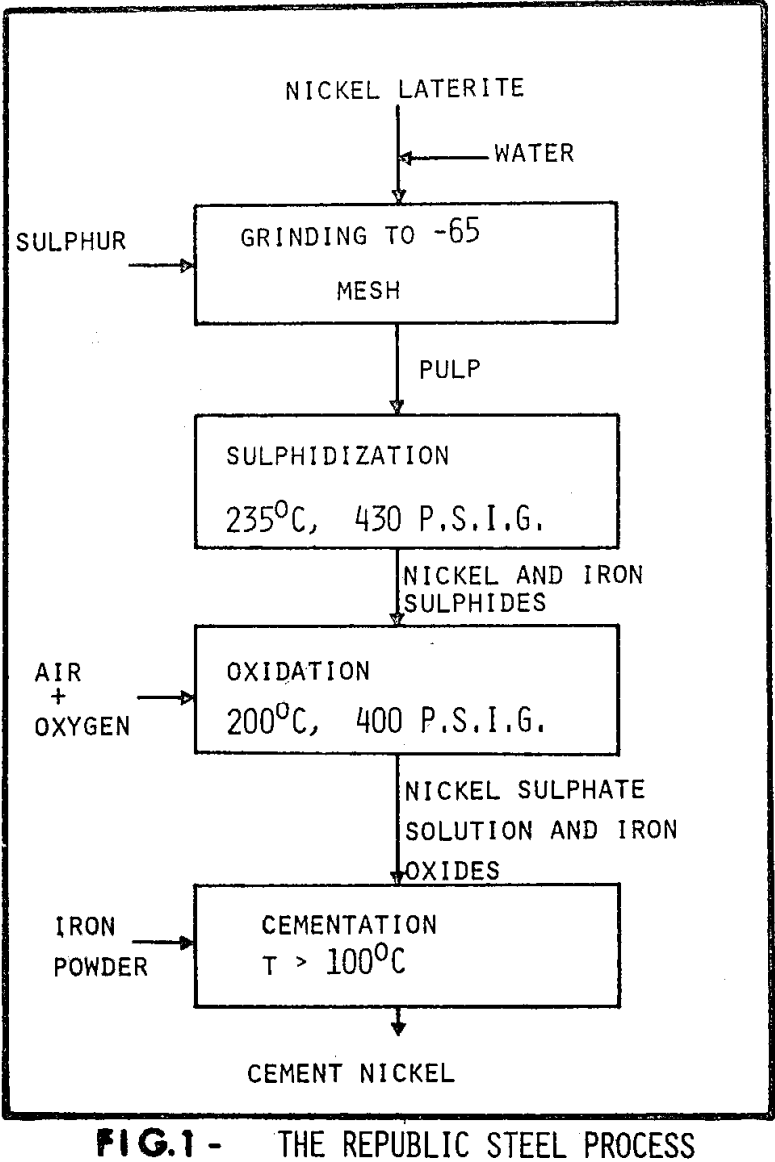
The cementation of the nickel onto iron in the process is a fundamentally important step as the efficiency of the whole recovery relies on it. The overall cementation reaction occurring may be described as :
Ni++ + Fe° = Ni° + Fe++
From thermodynamic considerations essentially complete removal of nickel from solutions is to be expected within the temperature range 298 to 473K if equilibrium can be achieved for this reaction. However, experimental work has shown that equilibrium is rarely, if ever, approached. Although thermodynamically viable, the cementation process is limited by kinetic factors.
General Model for Precipitation Reactions
The interpretation of results obtained from experiments in cementation reaction kinetics, requires the establishment of a qualitative model involving all possible successive mechanism stages which take place during the course of the reaction.
In general terms, a cementation reaction such as one represented by equation 4 can be considered to take place via the following steps :
(i) Transport of ions of the cementing species (Mm+) from the bulk of the solution to the deposition sites,
(ii) Conduction of electrons of the precipitant metal (N) from the dissolution sites to deposition sites,
(iii) Reduction and incorporation of atoms (M) into a crystal lattice of deposit (including loss of water ligands).
(iv) Release of ions of the precipitant (Nn+) into the solution (and hydration).
(v) Transfer of precipitant ions (Nn+) released from the vicinity of the reaction surface into the bulk of the solution.
Once the rate limiting conditions have been identified, it is usually possible to operate the process in such a manner as to maximize the rate. For example, increases in the overall rate resulting from increases in temperature are much more pronounced in systems where the rate is limited by the surface-chemical processes. Similarly, when mass transfer is rate limiting, an increase in the agitation in the system will bring about an increase in the rate.
Experimental Equipment and Method
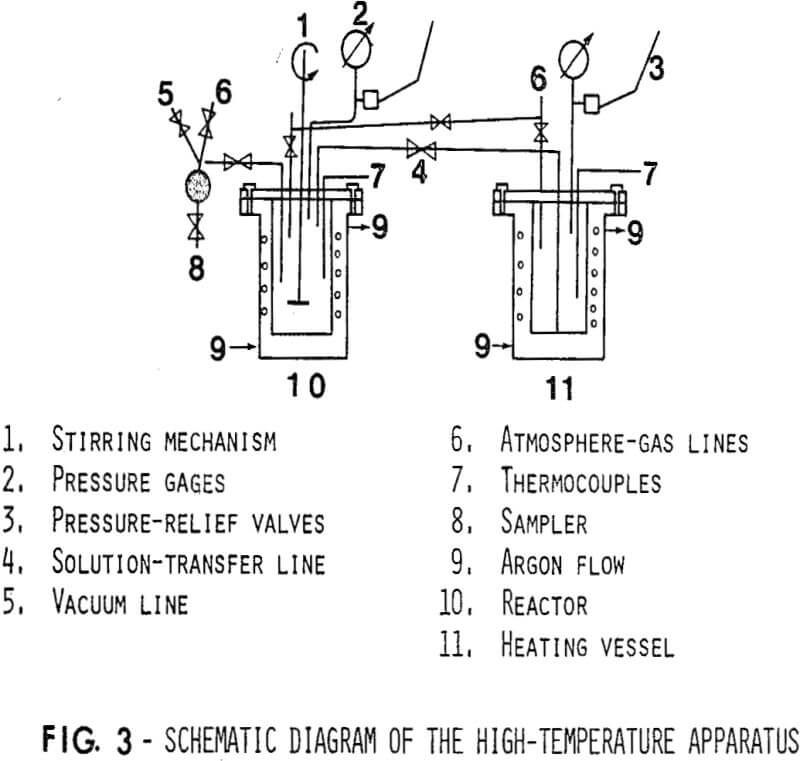
The experimental equipment consists of two two-litre capacity autoclaves provided with independent heating, pressure, and vacuum systems, and interconnected by two sets of high-pressure tubing with isolating ball valves. Its design incorporates some rather flexible operational features which allow the study of solid-liquid reactions at temperatures up to 200°C, and pressures ranging from sub-atmospheric to 250 p.s.i.g.
The autoclaves themselves are seam-welded titanium vessels in stainless steel jackets. All parts which might come into contact with the test solution are either titanium or are coated with Teflon. This precaution was considered necessary in order to ensure that in this series of tests the only source of iron in the system was the precipitant disc surface.
Unless otherwise specified, the experimental conditions were:
- Initial solution volume : 1500 ± 5 cm³
- Atmosphere in the reactor : oxygen-free nitrogen
- Temperature : 100 + 1°C
- Exposed area of disc precipitant : 25.65 cm²
- Rotation speed : 90 r.p.m.
- pH : natural (between 5.0 and 6.0)
- Pressure : steam pressure + 35 p.s.i.g. = 49± 1 p.s.i.g.
- Sample volume : usually 10 cm³
- Initial Ni concentration in solution : 10 ppm
- Initial Fe++ and impurity levels in solution : below measurable limits.
Results
The effect of variations in the disc rotation speed on the rate of precipitation of nickel onto iron at 50, 100 and 150°C. The deposits obtained under these conditions were very smooth and dense, and were found to be metallic nickel by X-ray analysis. The shapes of the experimental curves indicate the existence of a mixed-control mechanism with diffusion becoming more important at lower rotation speeds and at higher temperatures. At higher rotation speeds and lower temperatures, the data suggest that the chemical contribution to the overall rate becomes most significant since the rate constant becomes almost independent of the disc rotation speed.
It was plotted the rate curves obtained from a series of tests carried out under different oxygen partial pressures while maintaining the total pressure on the system constant. In some tests, two rate periods were observed : an initial period in which transport to an essentially “clean” disc takes place, and a second, or enhanced rate period in which the transport of reactants to the reaction surface is increased by the presence of rough deposits.
The specific rate constants are plotted against the initial nickel-ion concentration in solution which shows that the rate constant decreases asymptotically as the initial-nickel ion concentration is increased. The appearance of the deposits obtained from these experiments varied from coarse, very porous, blackish-red dendrites, at the lower initial nickel-ion concentrations, to very smooth, dense, light-brown cements at higher initial concentrations.
A plot of the percentage of nickel deposited from solution as a function of time indicates that nickel recovery achieved is inversely proportional to the initial nickel-ion concentration in solution. However, the chemical analysis of the cement deposits, indicates that the nickel content increases significantly with increasing initial-nickel-ion concentration in solution and that the mass ratio of nickel to iron in the deposit is greatly increased as the initial-nickel-ion concentration goes from 5 to 50 ppm.