Gold Refining Methods
The processes used for Gold Refining are as follows: Volatilisation. Oxidation (a) by air blowing or roasting. (b) by “ bessemerising” (c) by nitre. (d) by metallic oxides. (e) by cupellation. Chlorination. Sulphurisation. The use of iron or carbon. The method to be used depends partly on the composition of the bullion, and partly on […]
Gold Melting Furnaces: Key Features and Applications

The furnace used for melting gold bullion is of simple construction. It may be round or square, with walls consisting of an outer layer of ordinary brick and an inner layer, at least 4 inches thick, of the best firebrick. There is often a complete outer casing of iron, which is useful in keeping the […]
Methods of Testing Cyanide Solutions

The method given above is difficult to apply when solutions containing soluble cyanides of zinc and other metals require to be titrated. “ A white flocculent precipitate occurs at a certain stage, probably consisting of simple (insoluble) cyanide of zinc, formed by decomposition of the soluble double cyanide: K2ZnCy4 + AgNO3 = KAgCy2 + ZnCy2 […]
How pH Affects Cyanide Decomposition & Use Alkalinity to Preserve Cyanide
Since acidity of the ore causes decomposition of the cyanide, an obvious method of reducing the loss is to add alkali in some form. Before doing this, the free sulphuric acid and soluble salts may be removed by leaching with water, and then a solution of caustic soda or lime is run on to the […]
Potassium Cyanide Chemistry on Minerals & Metals

The ordinary gangue of most ores (silica and silicates of the alkalies and alkaline earths) exercises no direct influence on the cyanide solution. The carbonates of the alkaline earths are also probably without influence. The decomposing effects of sulphides of the heavy metals vary with the physical state of the sulphides. Metallurgists found that dilute cyanide […]
Potassium Cyanide Leaching with Gold and Mercury
These methods are described here for convenience, as being more intelligible after the chemistry of the process has been discussed. The necessity of the presence of oxygen has already been dwelt on above. It has, however, been frequently pointed out that in the interior of a mass of ore undergoing treatment the conditions are not […]
Oxidised Pyrite Leaching by Potassium Cyanide

When the pyrites occur in tailings which have been subjected to the action of the weather for some time before treatment, compounds are formed which are more prejudicial to the solution than the sulphides. Sulphide of iron, FeS2, is oxidised by air and water, ferrous sulphate and free sulphuric acid being formed, thus: FeS2 + […]
Zinc Box for Gold Bullion Precipitation
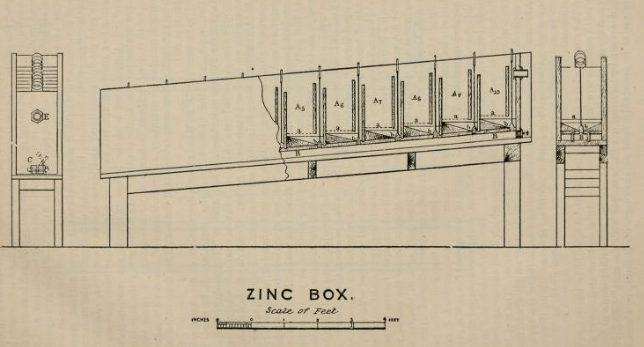
It was generally believed at one time that the precipitation of gold and silver by zinc was effected by simple displacement, according to the equation: 2KAuCy2 + Zn = K2ZnCy4 + 2Au Some considerations have been adduced, however, which throw doubt on this view. It is well known that a considerable excess of free cyanide must […]
Effect of Potassium Cyanide on Gold & Metals

The discovery that metallic gold is soluble in potassium cyanide came after studying the action of cyanides on plates of gold, and announced that they were slowly dissolved. Metallurgists pointed out that gold-leaf is dissolved by a dilute solution of the salt, and also showed that if the gold floats on the surface of the […]
Gold Vat Leaching
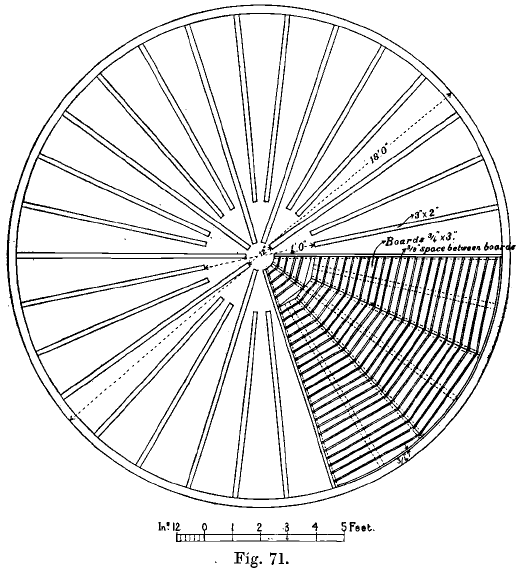
Gold Leaching done in round vats, constructed of wood, concrete, or iron and steel. If wood is used it is covered by a coating of paraffin paint, or by a mixture of asphaltum and coal-tar. Concrete and brick vats are not now advocated owing to their great cost and less convenient working, and wooden vats suffer more […]
