Table of Contents
This powerful CN- detoxification agent is prepared by mixing concentrated sulfuric acid and concentrated H2O2.
H2O2 + H2SO4 → H2SO5 + H2O
The formation of Caro’s acid is instantaneous and is accompanied by the evolution of heat, primarily due to the heat of dilution of sulfuric acid. Because of it’s tendency to decompose to oxygen and sulfuric acid, Caro’s acid is not stored, but is used immediately at the generation site. Using a 2.5/l mole ratio of H2SO4/H2O2, the resulting solution contains 25% Caro’s acid, 3.5% H2O2, 57% sulfuric acid and 14.5% water.
The Caro’s acid reacts with the CN- within one to two minutes:
H2SO5 + CN- → H2SO4 + CNO-
The H2O2 remaining after the formation of Caro’s acid reacts with CN- over a longer time period (>15 minutes)
H2O2 + CN- → H2O + CNO-
Process Description
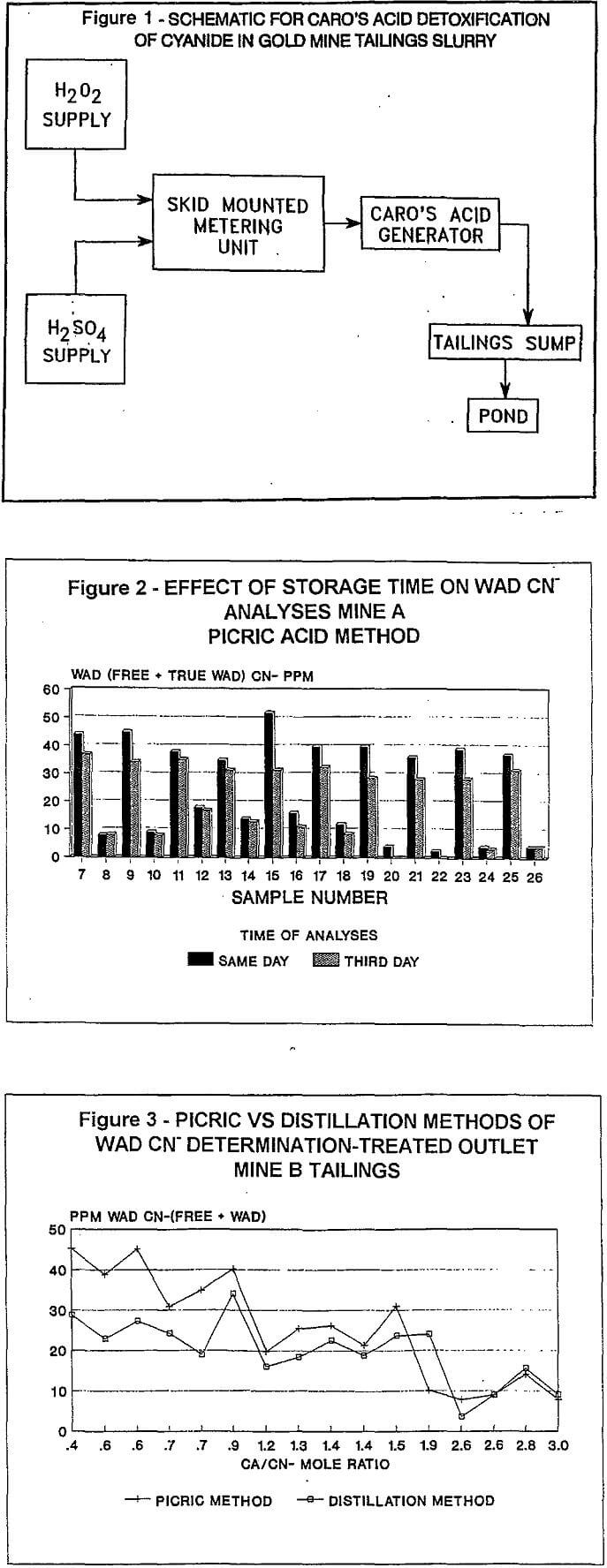
The FMC continuous Caro’s acid generator consists of a (1) a skid mounted unit containing metering pumps and a process loop controller. (2) a static-mixer reactor (3) a water flood system and (4) storage vessels for the hydrogen peroxide and sulfuric acid. Sulfuric acid and hydrogen peroxide break tanks are optional (Figure 1).
The static-mixer reactor is positioned over the tailings sump located several feet from the Caro’s acid skid unit. Stainless steel piping connect the sulfuric acid and H2O2 lines between the skid unit and the mixer-funnel. The tailings sump is located indoors at Mine “A” and outdoors at Mine “B”.
Sulfuric acid and hydrogen peroxide are pumped from the feed skid at a tightly controlled rate to the static-mixer reactor where the Caro’s acid is instantly formed. The Caro’s acid is immediately discharged into the tailings sump.
After the Caro’s acid and the tailings are mixed in the sump, the tailings travel through high density polyethylene (HDPE) lines to the tailings ponds. The detention time of the slurry in the HDPE lines for both mines is between 15-20 minutes.
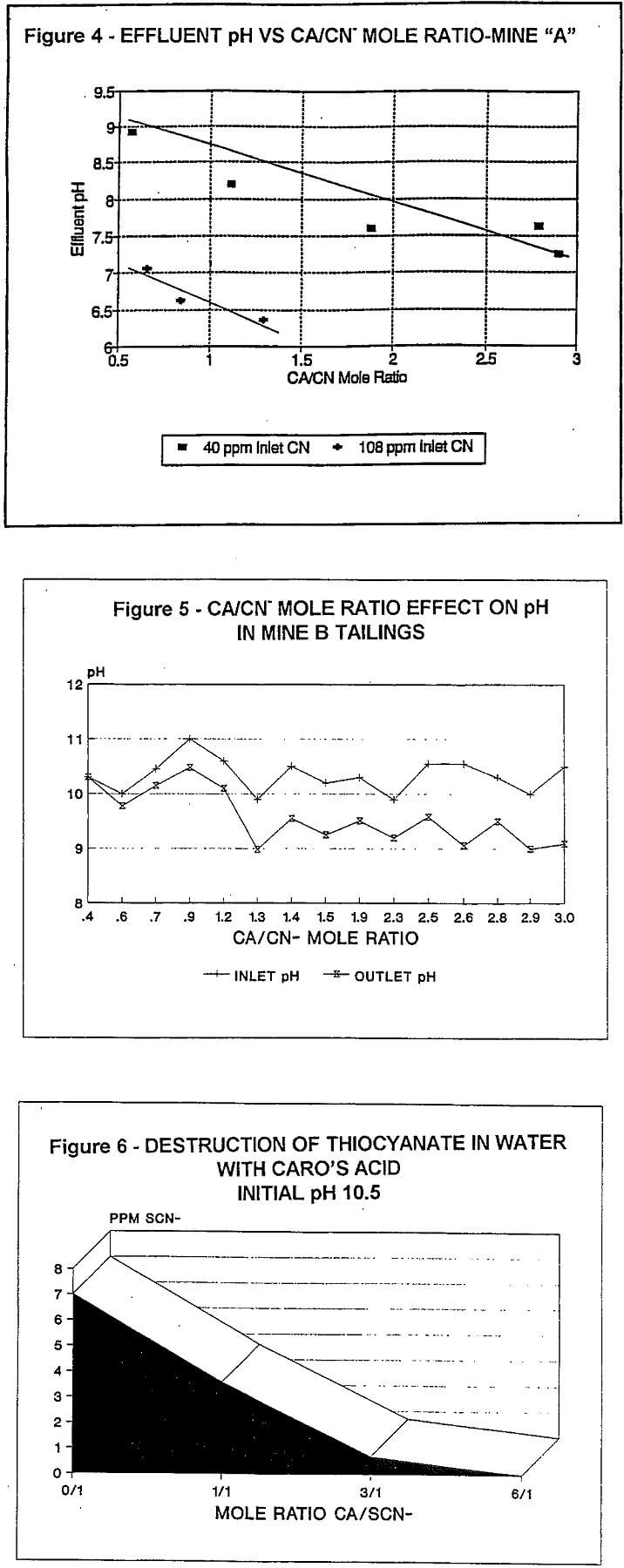
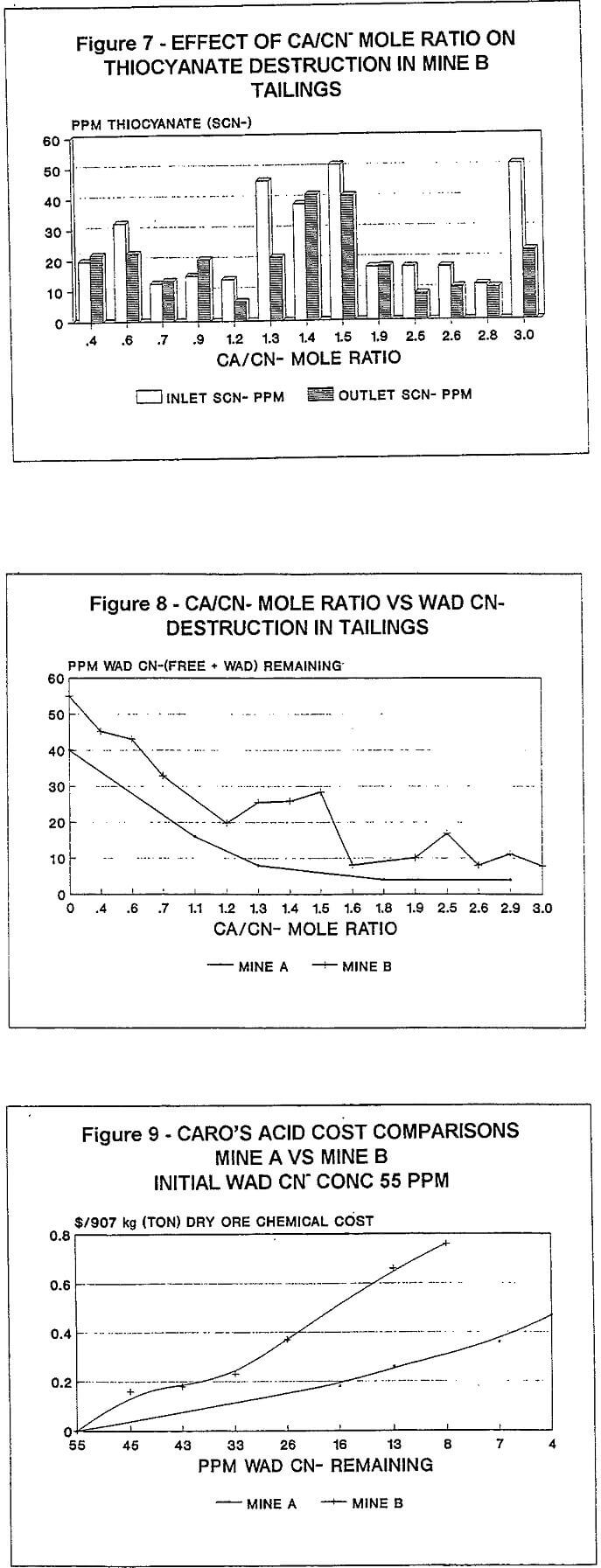
The term “WAD CN” as used in this report, is the sum of true WAD CN- plus free CN-.
Effect of Caro’s Acid On pH
The amount of pH drop when adding Caro’s acid will vary with the type of ore, amount of lime used in the cyanidation step, the initial and final WAD CN- concentrations and the ratio of Caro’s acid (CA)/CN-.
With Mine “A” tailings, at a CA/CN- mole ratio of 1/1, an inlet WAD CN- concentration of 40 ppm and an initial pH 10.5, the effluent pH was lowered to 8.8 (Figure 4).
With Mine “B” tailings, at a CA/CN- mole ratio of 1/1, an initial WAD CN- level of 55 ppm and a pH of 10.8, the pH was lowered to pH 10.3 (Figure 5) .
These two results illustrate significant differences in final pH between the two mines at equal mole ratios of CA/CN- and similar starting concentrations of WAD CN- (40-55 ppm) . The differences are probably due to the amount of lime used and/or different ore characteristics.
When the initial WAD CN- concentration was increased to 108 ppm in Mine “A” tailings, the pH dropped to 6.6 (Figure 4). Since WAD CN- dissociates more readily as the pH is lowered, the drop in pH can be an advantage in achieving lower levels of WAD CN-.
A concern with reducing the pH however, is the possible release of HCN into the atmosphere creating a health hazard. Surprisingly, unacceptable releases of HCN did not occur at Mine “A” where the pH dropped to a low of 6.6. HCN concentrations over the sump and throughout the sump building did not exceed 2 ppm. The minimal HCN levels were believed due to the very rapid reaction of Caro’s acid with CN- and the mixing and flow patterns of the slurries. In mine “B”, HCN release was less of a concern, since the average pH after Caro’s acid addition was above 9 and the tailings sump was located outdoors. As expected, measurements taken around the base of the tailings sump registered non-detectable levels of HCN.
Since process conditions e.g. recycling of low pH pond water into a CN- reclaim water tank may result in HCN release in the mill, extra lime or other alkali should be available to raise the pH should it be needed. As a further precaution, periodic HCN measurements should always be made in confined areas surrounding the detoxification process.