Carbon adsorption was considered as the most adequate method for recovery of silver from thiourea leach solutions. For this reason it was decided to examine the characteristics of adsorption of silver on activated carbon. Coconut hard shell activated carbon was used for this study.
Experimental
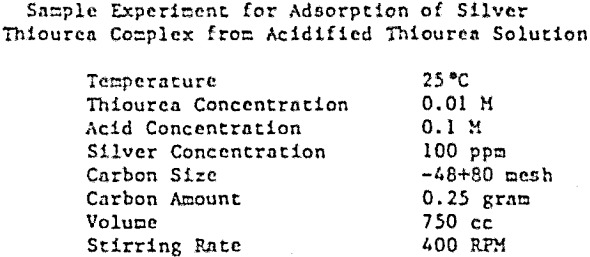
The carbon, coconut hard shell, was pulverized and dry seived into monosized fractions in the range +48 to -150 mesh. The moisture content after drying at 110°C was less than 0.1% for all sizes, and the -48+80 mesh size fraction was used in all experiments conducted in this investigation. Silver thiourea complex, Ag(TU)2Cl, was prepared and dissolved in an HCl-thiourea solution to give a working stock solution of 2 gpl silver and 0.2 M thiourea. This solution was subsequently diluted to the desired concentration of silver and thiourea for each experiment with deionized water or hydrochloric acid solution.
Results
The adsorption rate of silver thiourea complex was studied as a function of pH, thiourea concentration, temperature, and ratio of silver to carbon present. The effect of oxygen was also examined, and the loading isotherms were determined at 25 C, 35 C, and 45 C.
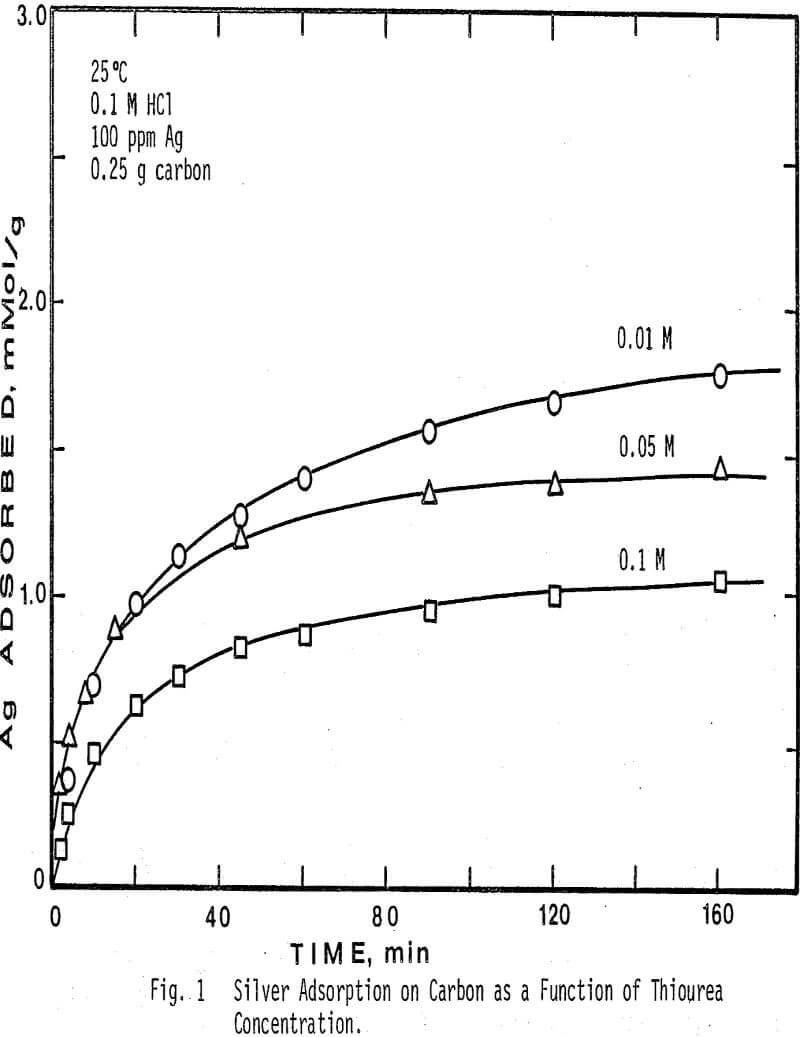
The concentration of free thiourea in solution was varied from 0.01 M to 0.1 M. Note that when a higher concentration of thiourea is present, less silver is adsorbed from solution. Since activated carbon is known to adsorb organic compounds from aqueous solution, this result would be expected, because thiourea will compete with the silver complex for adsorption sites on the surface of the carbon, and thus a higher concentration of thiourea will depress the amount of silver adsorbed. In addition, the initial rate of adsorption of silver is seen to be lower in the more concentrated thiourea solutions.
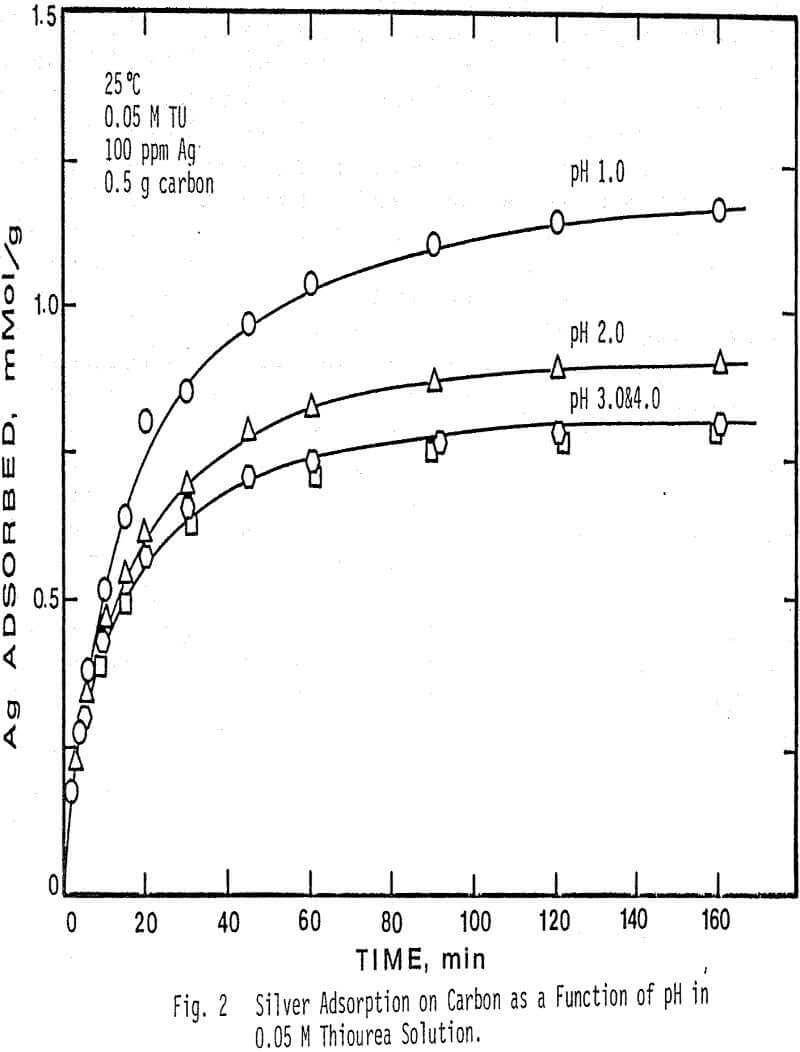
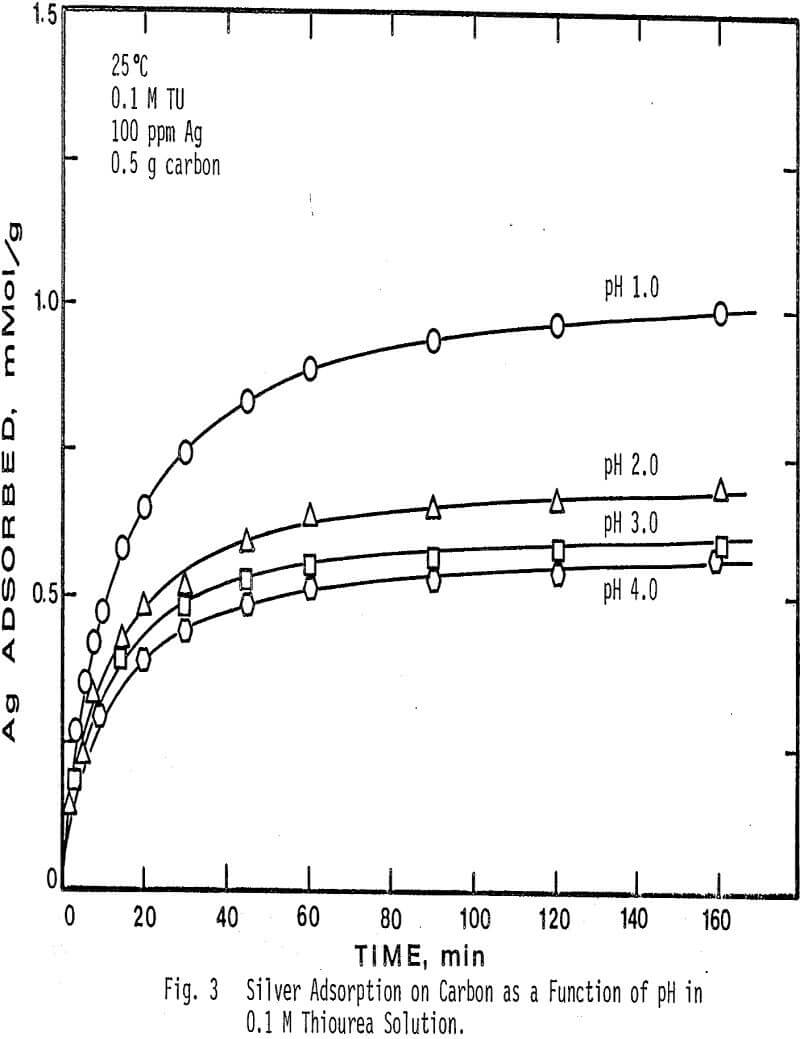
The effect of pH on the adsorption of silver from solution was determined at four different pH values and two different thiourea concentrations. pH values higher than 4.0 caused decomposition of the silver complex and low adsorption.
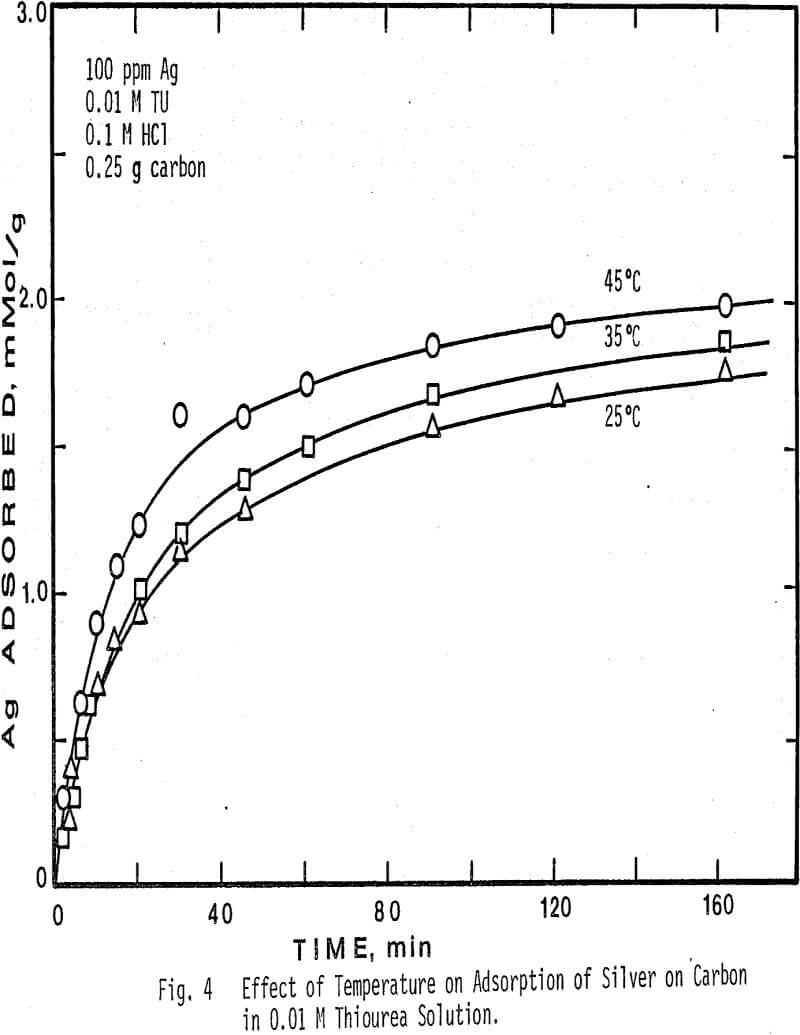
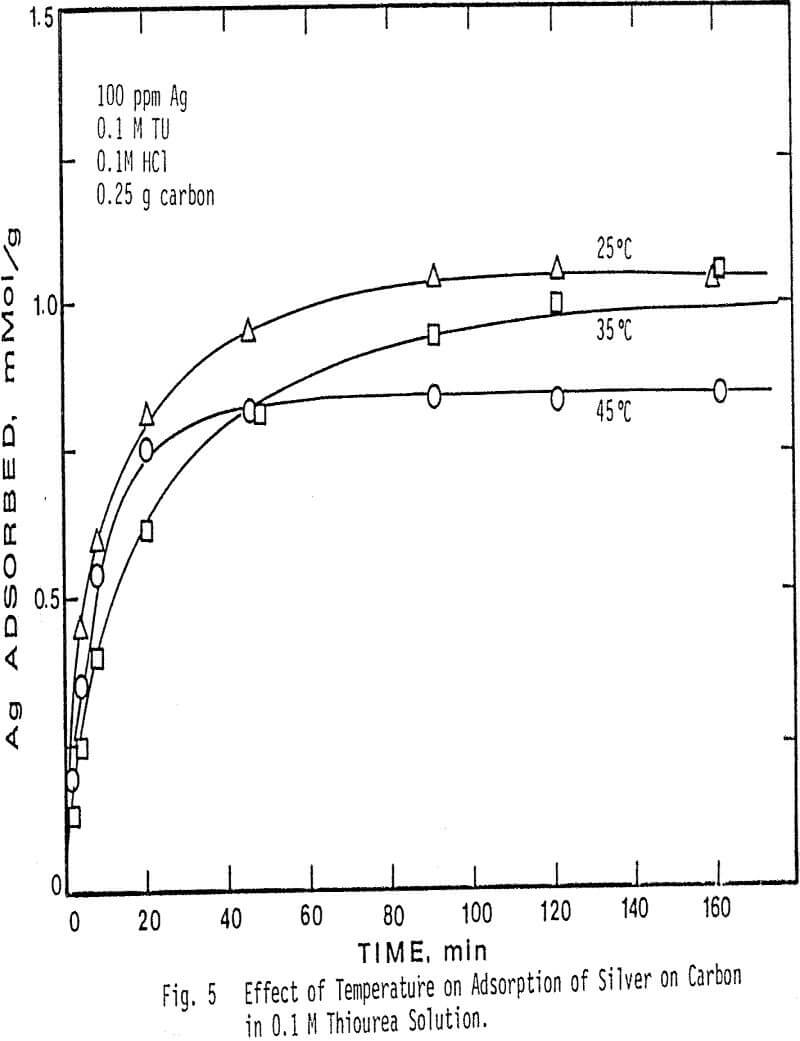
Higher temperatures than 45°C were not investigated, due to the instability of thiourea at temperatures above this value. The results show that at higher temperatures in more concentrated thiourea solution the adsorption of silver decreases. Also note that at a fixed temperature, the amount of silver adsorbed per gram of charcoal is greater in 0.01 M thiourea solution than in 0.1 M thiourea solution.
Rate of Reaction
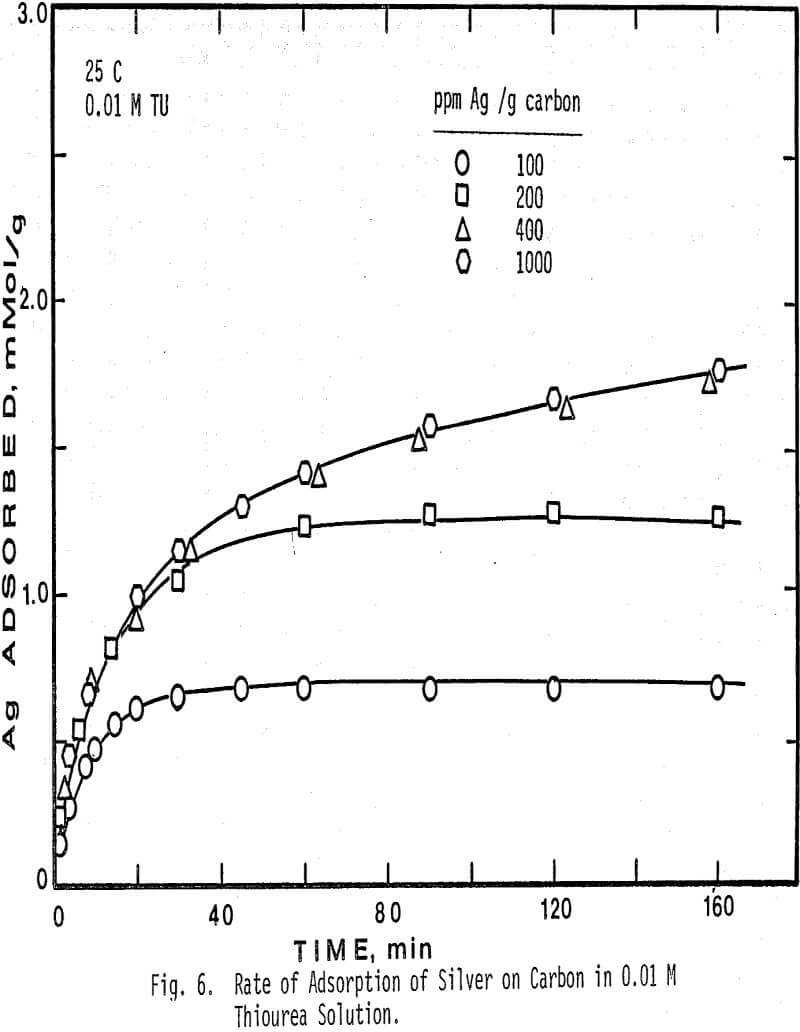
The results indicate that the initial rate as determined by this method is a function of the initial ratio of silver to carbon, and is first order with respect to silver concentration in solution. The effect of the ratio of ppm Ag/g carbon on the initial rate of adsorption is that at high ratios the rate of adsorption is low, and at low ratios the initial rate is fast for constant silver concentration. Thus the initial rate is primarily dependent on the initial silver concentration in solution and the total carbon area.
In order to understand better the process of adsorption of silver from acidified thiourea solution onto activated charcoal, several theoretical aspects of adsorption must be considered. Adsorption is a surface phenomena and in aqueous solution involves the concentration or accumulation of chemical substances at a solid-liquid interface. Adsorption of a chemical species from solution onto the surface of a solid can occur in one of two ways.
Silver adsorption on activated charcoal from acidic thiourea solutions is faster in more dilute thiourea solution. The amount of silver adsorbed is also larger in dilute thiourea solution.