Table of Contents
This is an interesting development of the old decantation process, and while it contains the same principle, of displacement by successive dilutions, the action is much more rapid, the same result is attained by the use of considerably less barren solution and it is continuous in action needing little power and practically no attention.
For its application a succession of Dorr thickeners is used, the series usually consisting of four. Each succeeding thickener is placed at a slightly higher elevation than the preceding one so that the clear overflow may be able to flow back by gravity. There are thus two separate currents of fluid running in opposite directions, the pulp after the dissolving treatment flows toward the residue discharge end of the series while the clear solution overflow runs backward from the discharge toward the treatment and milling end of the plant.
The system is usually, though not necessarily, supplementary to some system of continuous agitation dissolving treatment. The slime pulp from the milling department goes first to a thickener, the clear overflow from which being the highest in value of any in the plant forms the pregnant solution for precipitation. The thick underflow is raised by a diaphragm pump to the series of continuous agitators and, as it enters, is diluted to the desired degree with solution overflowing from No. 1 washing thickener. The pulp, when the dissolving treatment is finished, passes continuously into No. 1 washing thickener, being diluted on its way by the overflow solution from No. 2 washing thickener. The underflow from No. 1 washing thickener is raised by a diaphragm pump to the level of No. 2 where it meets the stream of solution overflowing from No. 3. The thick underflow from No. 2 is raised to the level of No. 3 where it is diluted with barren solution and also with the overflow solution from No. 4. The underflow from No. 3 is raised to No. 4 and diluted with water. The underflow from No. 4 goes to the dump but if necessary may be first passed through a dewatering filter.
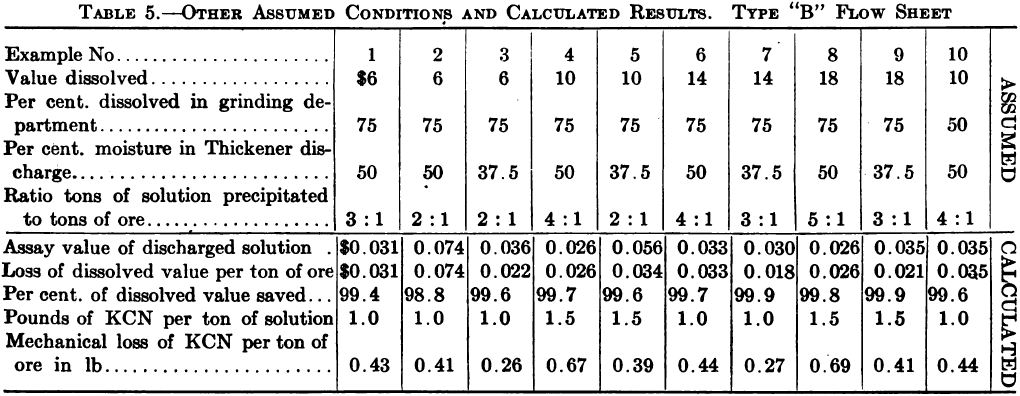
The following typical flow sheet and calculations for dissolved. value loss are taken from a pamphlet issued by the Dorr Co.
Regarding the assumption in these calculations that 75% of the whole metal dissolved goes into solution during milling, this is probably about correct for a gold ore, but for a silver ore it would in the writer’s experience be nearer the facts to assume a dissolution during milling of only 15% to 25% of the total silver dissolved.

Calculations for Dissolved Gold Value Loss
Conditions Assumed:
(a) 100 tons of ore per day crushed in cyanide solution.
(b) Discharge from all Thickeners with 50% moisture.
(c) $10.00 value dissolved per ton of ore.
(d) 75% in mill and 25% in Agitators.
(e) 400 tons of solution from Thickener V precipitated to $0.02.
(f) Agitation with a dilution of 2 of solution to 1 of solids.
(g) Let V, W, X, Y and Z represent value in dollars per ton of solution discharged from the respective Thickeners.
Equating out of and into each Thickener:
(1) 100V + 400V = 500W + (0.75 X $10.00 X 100).
(2) 100 W + 600W = 500X + 100W+(0.25 X$10.00X 100)+100V.
(3) 100X + 500X = 100W + 500Y.
(4) 100Y + 500Y = 100Z + 100X + (400 X 0.02).
(5) 100Z +100Z = 100Y + 100 tons of water value $0.00.
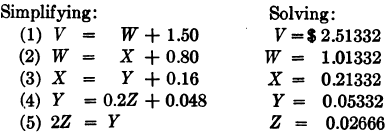
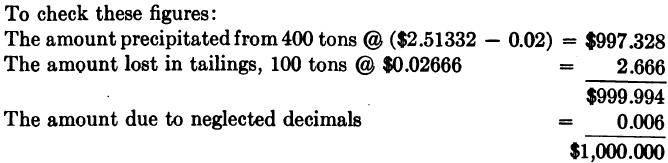
The amount dissolved 100 tons @ $10.00 = $1,000.00.
From the foregoing the following results are deduced:
Assay value of the pregnant solution, i.e., value of V = $2.51332
Assay value of the discharged solution, i.e., value of Z = 0.02666
Loss of dissolved value per ton of ore, = 0.02666
Dissolved value saved 99.7 %.
Calculation for Mechanical Gold Loss of Cyanide
Conditions Assumed:
(a) Neglect the Cyanide consumption throughout the system.
(b) Strength of Cyanide per ton of solution 1.0 lb.
(c) Let V, W, X, Y and Z represent the strength in pounds of Cyanide in Solution discharged from the respective Thickeners.
Equating out of and into each Thickener:
(1) V = 1.0.
(2) 100W + 600W = 100W +100V + 500X.
(3) 100X + 500X = 100W + 500Y.
(4) 100Y + 500Y = 100Z + 400V + 100X. .
(5) 100Z + 100Z = 100Y + 100 tons of water.
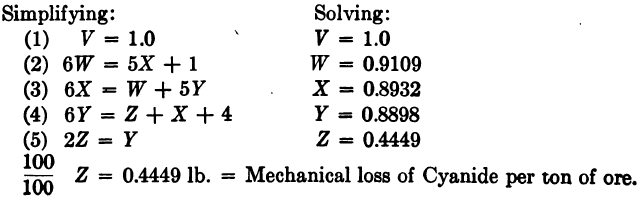
The two requisites for efficient working of the system are a sufficient volume of precipitated solution and a perfect precipitation yielding a tail solution that is for practical purposes barren. It is possible under these conditions to get a very satisfactory elimination of the dissolved precious metals from the residue.
Regarding elimination of the cyanide it is not quite so satisfactory because, as in all other washing processes, the quantity of final water wash available is limited by the daily tonnage of moisture eliminated from the plant, as otherwise the stock solutions would soon increase to an unmanageable bulk. In the case of gold ores where a weak cyanide solution can be used with effect the loss of cyanide in the residue is inconsiderable, but for silver ores where a treatment solution of 0.1% or over is employed it will often be found profitable to use a filter of some kind before discharging the residue from the last thickener to the dump.
It has often been observed, when working this process, that although the dissolving action in the treatment tanks may have apparently ceased before the pulp enters the washing thickeners, additional small amounts of precious metal seem to pass into solution during the washing out process, corroborating the theory already alluded to of the efficacy of successive washes for the dissolving, process, a tentative explanation of which has already been given.
Another phenomenon, rather difficult to explain, is also sometimes in evidence in the use of this process. Even in cases where little or no dissolving action is observed in the successive thickeners in which cyanide solution is used as the diluent yet when the pulp reaches the last thickener and is diluted with water marked dissolution of the metals seems to take place. This may perhaps be due to the increased activity of the ions in a less viscous medium than is afforded by the stock solution. On the other hand it seems possible that the dissolving effect may be only apparent and that in reality the result may be due to a liberation of pregnant solution previously adsorbed by the colloidal constituents of the pulp. Such a liberation might conceivably be brought about by the changes in the properties of the electrolyte due to excessive dilution with water. One such change might be traced to a decrease of lime in solution sufficient to cause the curds or aggregations of clayey matter to disintegrate into their original units liberating soluble matter which had been entangled with them. If it be asked why a similar action is not displayed when making washed assay samples for the determination of these various points, the answer might lie in the fact that such washed assay samples are now almost universally prepared on laboratory vacuum filters, so that they are not subjected to the same conditions as obtain in the working of the plant.
