Without a meter, acidity or alkalinity determinations of water for and solutions used in plants treating gold and silver ores are of importance. Methods range from simple tests with litmus papers or phenolphthale in solutions to pH determinations.
pH Determination
The pH value indicates with a high degree of accuracy the amount of active acidity or alkalinity in contrast to the total acidity or alkalinity as determined by ordinary titration methods. The symbol stands for the logarithm of the reciprocal of the hydrogen-ion concentration H+, and on this account the higher the numerical value on the pH scale the smaller the number of free hydrogen ions in solution, and vice versa. Furthermore, it will be noted that, since the relationship is logarithmic, each successive pH number represents a tenfold change in the hydrogen-ion concentration. Pure de-aerated distilled water is neutral in its chemical reaction, which means that it is neither acid nor alkali and that its number of H ions is equal to its content of OH ions. It has also been determined by analysis that the degree of ionic dissociation of pure water, or, in other words, its hydrogen-ion concentration, is 0.0000001 (one ten-millionth) gram per liter (1000 cc). Therefore by the definition given in the previous paragraph, its pH value would be determined as follows: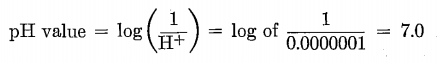
This pH 7.0 value is therefore the neutral point of the pH scale at 22°C. This neutral point rises and falls inversely with the temperature, and at 18°C. it is 7.1.
A 0.1 normal solution of hydrochloric acid contains by definition 0.1 gram ionizable hydrogen per liter, so that if completely ionized the solution would contain 0.1 gram hydrogen per liter. From electrical measurements, however, it is known that at 18°C. only 91.4 per cent hydrochloric acid is dissociated into ions. The balance remains in the solution as HCl molecules.
Since only 91.4 per cent is ionized, it contains
0.1 x 91.4/100 = 0.0914 gram H+ per liter
Therefore the pH value of 0.1N HCl is
log of 1/0.0914 or log 10.94 = pH 1.04
By a similar calculation it can be shown that a 0.1 N solution of acetic acid, whose 0.1 gram per liter ionizable hydrogen is dissociated only to the extent of 1.36 per cent, has a pH of 2.86. In other words, 0.1 N HCl contains almost 70 times the number of active hydrogen ions as 0.1N acetic acid, .which by ordinary chemical titration methods is of the same strength.
Similarly, 0.3N solutions of sodium bicarbonate (NaHC03), sodium carbonate (Na2C03) and sodium hydroxide (NaOH), which all have the same alkalinity when measured by ordinary chemical titration methods, have pH values of the order of 8.40, 11.60, and 13.00, respectively.
Because of this difference in ionization, different acids and alkalis are designated as weak or strong. As applied to corrosion, pH values measure the intensity of the corrosive action, while total acidity by titration measures the amount of corrosion which will occur before the acid is exhausted.
The method of pH determination is particularly useful where the acidity or alkalinity is so slight as to be below convenient titration range. One method of measurement is to add specific “indicators” which show characteristic color changes at certain pH values and to compare these colors with standards in the form of solution or color charts. One instrument largely used for this purpose is the LaMotte roulette hydrogen-ion comparator, where a large number of standard solutions may be viewed alongside the sample against a fixed light source.
Another device makes use of specially treated paper enclosed in plastic containers. Pieces are torn off, immersed in the solution, and the color change compared with charts on the containers (Beckman type).
The most accurate device, however, is the pH meter, an electrometric instrument which measures the pH directly against a standard calomel electrode. These instruments, which are made by the Leeds and Northrup Company of Philadelphia, Pa., the National Scientific Laboratories of Pasadena, Calif., and others, are carried by all leading supply houses.
