This report presents the results of a Bureau of Mines laboratory investigation to ascertain if the precipitation of calcium vanadate from low-tenor alkaline leach solutions can be practically employed for producing marketable technical-grade oxide (red cake) or ammonium metavanadate (AMV). The research was undertaken during the development of an autoclave process for extracting vanadium from ferrophosphorus. The alkaline leach solution from autoclave treatment contained only 7.3 grams V2O5 per liter, while red cake production requires a solution containing 20 grams V2O5 per liter and AMV production requires solutions containing 100 grams V2O5 per liter.
Specific attention was given to (1) the study of selective precipitation of calcium vanadate and (2) the means of dissolving calcium vanadate in acid, sodium carbonate, and sodium bicarbonate solutions to obtain vanadium-enriched solutions from which either red cake or AMV could be recovered.
Solvent-extraction techniques have been employed by various investigators to recover vanadium from low-tenor acidic solutions ; and among other methods, the literature refers to precipitation of ferrous, lead, and calcium vanadates. The precipitation of calcium vanadate was deemed the most promising method, because previous research had indicated that incremental additions of calcium chloride would first purify the autoclave leach solution of phosphorus by the formation of tricalcium phosphate and would then precipitate calcium vanadate.
Little pertinent information about calcium vanadate was found in the literature, and no important commercial uses have been developed, although there was an unsuccessful attempt to employ calcium vanadate of high lime content as a raw material for the production of ferrovanadium. Apparently, use of a calcium vanadate of lower lime content was not tried. A patent was granted to Cole and Breitenstein on a method for recovering vanadium from alkali metal vanadate solutions by adding a mixture of calcium chloride and calcium hydroxide. According to a patent granted Porro, Eding, and Wilder for recovering vanadium and molybdenum from low-grade ores, lime was added to a leach solution to precipitate calcium vanadate; the precipitate was extracted with sulfuric acid, and the extract was boiled to precipitate red cake.
Both these patented calcium vanadate precipitation procedures are empirical and fail to relate pH and calcium controls to product composition and, because they were not directly applicable to the highly alkaline autoclave solution, the research reported here was undertaken to determine the conditions required for precipitating calcium vanadate from synthetic sodium vanadate solutions and for dissolving the precipitates. The information was then applied to the processing of the autoclave leach solution.
Experimental Work
Materials, Equipment, and Procedure
Analytical reagent grade sodium metavanadate (NaVO3·H2O), CaCl2, CaO, and NaOH were used in the investigation. The vanadate was dissolved in water to obtain the desired concentration of V2O5 in solution. When precipitating with CaCl2, the CaCl2 was dissolved in a minimum of water then added to the vanadate solution. The pH was adjusted by adding dilute NaOH solution. When precipitating with CaO, the CaO was slaked to form Ca(OH)2 and added as a milk of lime slurry.
The reactions were made in glass beakers by heating the solutions to between 85° and 95° C on an electric hotplate. Agitation was obtained by motor-driven, glass-rod agitators. A pH meter accurate to the third decimal place and sensitive enough to detect subtle differences or changes in pH levels was used. The meter was equipped with special electrodes correcting for high sodium ion and temperature when continuously immersed in a solution heated to between 85° and 95° C. The meter was recalibrated as required during a test after each two-to-threefold change in the pH reading.
Synthetic sodium vanadate solutions, containing 8 to 100 grams V2O5 per liter, were used in a series of exploratory tests to define conditions for precipitating calcium vanadate with CaCl2 and Ca(OH)2. The information derived by precipitating calcium vanadate from synthetic solutions was then applied to autoclave leach solution of pH 9.7 which analyzed 7.3 grams V2O5, 3.0 grams Cr, and 4.9 grams CO3 per liter. Finally, the calcium vanadate precipitates were extracted with acid, soda ash, or sodium bicarbonate solutions to obtain enriched vanadium solutions suitable for processing for recovery of red cake or AMV.
Precipitation of Calcium Vanadate from Synthetic Solutions
The factors investigated in the first group of tests, employing calcium chloride, were the effects of pH, pH and the quantity of calcium, and vanadium concentration on the calcium vanadate composition and the completeness of precipitation. Preliminary tests indicated that pH affects the composition of the precipitate independently of the quantity of calcium present but that both pH and quantity of calcium must be held within certain limits to obtain a desired calcium vanadate composition and nearly complete precipitation. The effects of Ca(OH)2 on pH, composition, and completeness of precipitation were investigated in the second group of tests.
Precipitation With Calcium Chloride
The reactions for precipitating calcium vanadates of different CaO:V2O5 mole ratios using CaCl2 are given in the following equations :
2NaVO3 + CaCl2 ↔ CaO:V2O5 + 2NaCl………………………………………………………….(1)
2NaVO3 + 2CaCl2 + 2NaOH ↔ 2CaO:V2O5 + 4NaCl + H2O……………………………(2)
2NaVO3 + 3CaCl2 + 4NaOH ↔ 3CaO :V2O5 + 6NaCl + 2H2O………………………….(3)
2NaVO3 + 4CaCl2 + 6NaOH ↔ 4CaO:V2O5 + 8NaCl + 3H2O………………………….(4)
3(2CaO:V2O5) + 2NaOH ↔ 2(3CaO:V2O5) + 2NaVO3 + H2O………………………….(5)
3CaO:V2O5 + CaCl2 + 2NaOH ↔ 4CaO:V2O5 + 2NaCl + H2O…………………………(6)
Effect of pH on Precipitate Composition
To determine the effect of pH on the composition of the precipitated calcium vanadate, a 200-ml volume of sodium vanadate solution containing 29.9 grams V2O5 per liter was reacted with 14.6 grams CaCl2 (4 moles CaCl2 to 1 mole V2O5 ) which is equivalent to 200 percent of the theoretical CaCl3, based on precipitating 2CaO:V2O5 according to equation 2. The addition of CaCl2 to the vanadate solution, while agitating and heating at 85° to 90° C, lowered the pH of the solution from 8.6 to 5.4 and formed only a small quantity of white precipitate.
The NaVO3-CaCl2 solution then was treated further by titrating with small increments of a 100-ml dilute NaOH solution that contained a total of 10.5 grams NaOH, which is equivalent to 133 percent of the theoretical NaOH, based on equation 4. After each addition of caustic solution, agitation and heating at 85° to 90° C were continued until a constant pH reading was
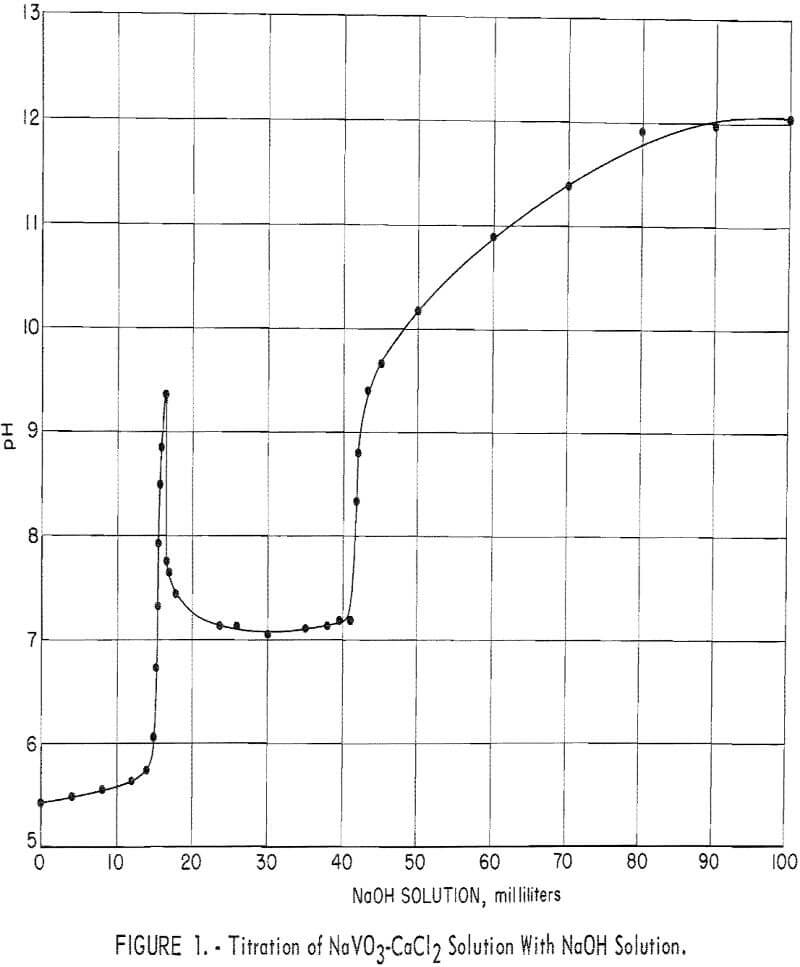
obtained. The time taken to attain a constant pH reading ranged from a few minutes to 2 hours. Small samples were taken to determine the precipitate composition and completeness of precipitation. Figure 1 shows titration results plotted with milliliters of caustic solution as the abscissa and the pH of the final solution as the ordinate.
A precipitation curve similar to that shown in figure 1, with only minor variations, was obtained by decreasing the CaCl2 added to the sodium vanadate solution from 200 to 100 percent of theoretical and titrating with NaOH solution.
Analysis of solution and precipitate samples showed that vanadium was precipitated in the following amounts;

Further additions of NaOH converted the 2CaO:V2O5 to 3CaO:V2O5, according to equations 5 and 3 and eventually the 3CaO:V2O5 to 4CaO:V2O5 by equation 6.
The inflection in the curve in figure 1, starting at pH 9.3, is attributed to the reaction between excess caustic in the solution and the accumulated 2CaO:V2O5 precipitate. The pH remained at 9.3 momentarily, then suddenly started a rapid decline to 7.8. The elevated pH appears to trigger the reaction to produce 3CaO:V2O5 and NaVO3 according to equation 5. This reaction was dominant until the decrease in pH leveled out at pH 7.1, when further addition of caustic converted the NaVO3 formed by reaction 5 to 3CaO:V2O5 by reaction 3.
Effect of pH and the Quantity of Calcium on Precipitate Composition and Vanadium Recovery
Synthetic sodium vanadate solutions containing 29.9 grams V2O5 per liter were reacted with 100 and 200 percent of theoretical CaCl3, based on forming 2CaO:V2O5 according to equation 2. The completeness of precipitation and the composition of the precipitates were determined for a pH range from about 6 to 12. The sodium vanadate solution was heated to between 85° and 95° C and the CaCl2 added. The NaOH solution then was added to obtain a constant prescribed pH, and the reaction continued at 85° to 95° C and constant pH for 1 to 2 hours to complete the reaction. The results of the individual tests are given in table 1.
The changes in the precipitate CaO:V2O5 mole ratios given in table 1 agree with the titration curve of figure 1. When 100 percent of theoretical CaCl2 was employed (2 moles CaCl2 to 1 mole V2O5, based on equation 2), the vanadium recovery increased from 10 to 93 percent as the test pH was raised from 5.9 to 8.0. Further change in the pH from 8.0 to 12.0 decreased the vanadium recovery from 93 to 58 percent. The CaO:V2O5 mole ratio remained constant at about 2 up to pH 9.0. At pH above 9.0, the 2CaO:V2O5 precipitate was converted first to 3CaO:V2O5, and second (incompletely) to 4CaO:V2O5. Since less than theoretical calcium was present for the precipitation of calcium vanadate above 2CaO:V2O5, some vanadium redissolved according to equation 5.
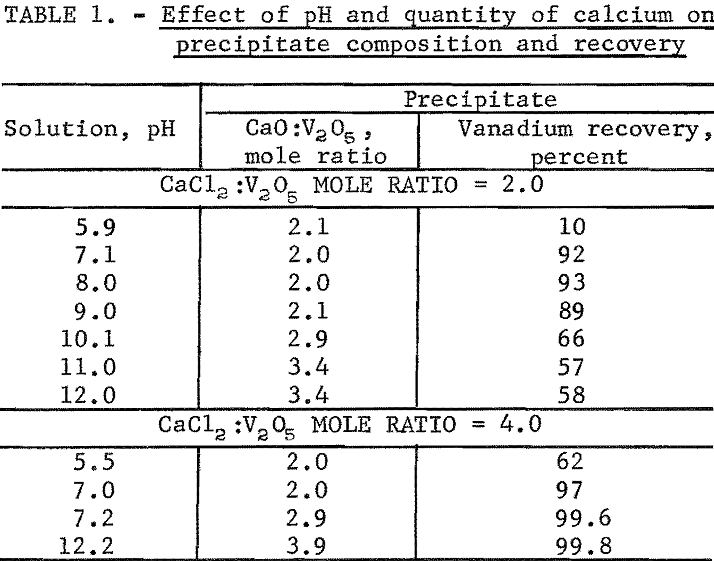
Increasing the quantity of calcium present to 200 percent of the theoretical amount in the second group of tests shown in table 1 increased the vanadium recovery at pH 7.0 to 97 percent. The precipitate CaO:V2O5 mole ratios increased from 2.0 to 2.9 in the tests made at pH 7.0 and 7.2. This change in the mole ratios corresponds to the main peak inflection in the curve of figure 1. The pH 7.0 point lies on the ascending portion of the curve and the pH 7.2 point on the descending (after the inflection). Since excess calcium was also present at pH 7.2 for the precipitate CaO:V2O5 mole ratio of 2.9, the resulting vanadium recovery was 99.6 percent. Finally, at pH 12.2, and a precipitate CaO:V2O5 mole ratio of 3.9, the vanadium recovery was 99.8 percent.
Effect of Vanadium Concentration on Completeness of Precipitation
Several tests were made to examine the effect of the vanadium concentration in the solution on the precipitation of dicalcium vanadate. The addition of 100 percent of theoretical CaCl2 to a sodium vanadate solution containing 100 grams V2O5 per liter precipitated 93 percent of the vanadium. When a sodium vanadate solution containing only 8 grams V2O5 per liter was reacted with the stoichiometric amount of calcium chloride, no calcium vanadate precipitated. However, vanadium recovery from the lean solution was virtually complete when 150 percent of theoretical CaCl2 and sufficient caustic to raise the pH of the vanadate solution from 6.0 to 8.0 were used. The CaO:V2O5 mole ratios of the precipitates were all near 2.0.
Precipitation With Milk of Lime
The theoretical reactions for precipitating calcium vanadates of different CaO:V2O5 mole ratios using Ca(OH)2 are given in the following equations:
2NaVO3 + Ca(OH)2 ↔ CaO:V2O5 + 2NaOH…………………………………………………(7)
2NaVO3 + 2Ca(OH)2 ↔ 2CaO:V2O5 + 2NaOH + H2O…………………………………..(8)
2NaVO3 + 3Ca(OH)2 ↔ 3CaO:V2O5 + 2NaOH + 2H2O…………………………………(9)
2NaVO3 + 4Ca(OH)2 ↔ 4CaO:V2O5 + 2NaOH + 3H2O………………………………..(10)
2CaO:V2O5 + Ca(OH)2 ↔ 3CaO:V2O5 + H2O………………………………………………(11)
To test the validity of these theoretical reactions, 200 ml of sodium vanadate solution containing 29.9 grams V2O5 per liter (pH 8.5) was titrated with 200 ml milk of lime slurry containing sufficient CaO to provide 200 per-cent of the stoichiometric requirement (7.4 grams CaO) to form 2CaO:V2O5 according to equation 8. Specifically, small-increment portions of the milk of lime slurry were added to the sodium vanadate solution maintained at 85° to 95° C, and, after each addition, agitation and heating were continued until a constant pH reading was obtained. Samples of the solution and precipitate were removed for analysis. The analysis and the proportion of the calcium vanadate precipitated are given in table 2.
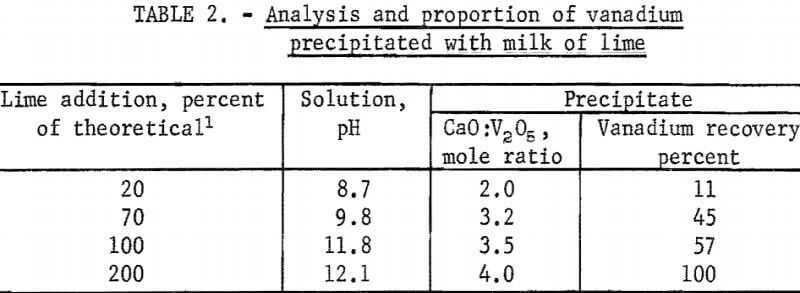
As indicated by the data given in table 2, the precipitation of calcium vanadates by reacting sodium vanadate solution at pH 8.5 with milk of lime involves several slow and complex reactions. The addition of 20, 70, or 100 percent of the stoichiometric amount of Ca(OH)2 required to precipitate 2CaO:V2O5 resulted in the precipitation of only 11, 45, and 57 percent of the vanadium as salts having CaO:V2O5 mole ratios of 2.0, 3.2, and 3.5, respectively. The addition of 200 percent of theoretical milk of lime resulted in 100-percent precipitation of the vanadium but as the 4CaO:V2O5 salt. The ineffectiveness of milk of lime as a precipitant of 2CaO:V2O5 was attributed to the slow and incomplete formation of this salt under the test conditions.
This latter assumption was checked by making a comparable precipitation test substituting calcium chloride equivalent to half of the milk of lime required for theoretical precipitation of 2CaO:V2O5. The synthetic sodium vanadate solution, containing 29.9 grams V2O5 per liter, was mixed with the calcium chloride solution and then with the remaining required calcium as milk of lime. This procedure, which maintained the solution in the pH 6.0 to pH 8.4 range, resulted in precipitation of 93 percent of the vanadium as 2CaO:V2O5
Precipitation from Autoclave Leach Solution
The precipitation curve of figure 1 established the conditions for precipitating dicalcium vanadate from synthetic sodium vanadate solutions when employing calcium chloride as precipitant and caustic soda for pH adjustment. The effectiveness of these conditions for treating more complex solutions was evaluated in a series of tests. The solution used in these tests was obtained by autoclave leaching of ferrophosphorus and purification to remove phosphorus. The solution of pH 9.7 analyzed 7.3 grams V2O5, 3.0 grams Cr, and 4.9 grams CO3 per liter. Preliminary testing established that the carbonate remaining in the autoclave leaching solution would be coprecipitated with the calcium vanadate.
Samples of the autoclave leach solution were agitated and heated to between 85° and 95° C and reacted with a quantity of calcium chloride calculated to provide 100 percent of the theoretical amount for precipitation of calcium carbonate and 110 to 130 percent of the theoretical amount for precipitation of dicalcium vanadate. The calcium chloride addition lowered the pH of the leach solution from 9.7 to 6.6. The pH then was adjusted with caustic soda solution to a pH of 8.0 to 8.5, and the reaction continued between 85° and 95° C for 1 to 2 hours. The vanadium recoveries increased from 98 to 99.5 percent as the calcium chloride additions were increased from 110 to 130 percent of the theoretical amount. The CaO:V2O5 mole ratios, after deducting the lime combined with the carbonate, averaged close to 2.0. Reagent consumption was 2.4 to 2.8 pounds CaCl2 and 0.5 pound NaOH per pound vanadium.
In a test variation, milk of lime was substituted for the caustic soda solution to adjust the pH after the calcium chloride addition. Comparable results were obtained, but the use of milk of lime for the control of pH within the narrow limits required for effective precipitation of dicalcium vanadate proved difficult.
Processing Calcium Vanadate
The primary objective in processing the calcium vanadate precipitates was to obtain enriched vanadium solutions containing 30 to 100 grams or more V2O5 per liter from which marketable grades of red cake or AMV could be recovered. Dicalcium vanadate was selected for conversion, because the higher lime salts were not easily processed. The higher lime salts, having CaO:V2O5 mole ratios ranging from 3 to more than 6 could be extracted satisfactorily, but agitation and pregnant solution recovery became difficult owing to an increase in pulp density as the lime increased. This was true when the calcium vanadate was extracted using sulfuric acid, sodium carbonate, or sodium bicarbonate but not when extracted with hydrochloric acid; however, in all cases the quantity of dissolving reagent required increased as the lime content of the salt increased.
The dicalcium vanadate recovered in the previous precipitation tests from synthetic sodium vanadate solutions was used to develop procedures for extracting vanadium. The best procedures were then applied to the dicalcium vanadate precipitate produced from the autoclave leach solution. The synthetic dicalcium vanadates were extracted in HCl, H2SO4, Na2CO3, and NaHCO3 solutions, and the precipitate from autoclave leach solution was extracted in H2SO4 and NaHCO3 solutions.
Dicalcium Vanadate Recovered From Synthetic Solution
The reactions used for extracting the vanadium from dicalcium vanadate in acid and alkaline solutions are given in the following equations:
3(2CaO:V2O5) + 12HCl ↔ H4V6O17 + 6CaCl2 +4H2O………………………………………….(12)
3(2CaO:V2O5) + 6H2SO4 ↔ H4V6O17 + 6CaSO4 + 4H2O……………………………………(13)
2CaO:V2O5 + 2Na2CO3 ↔ NaVO3 + Na3VO4 + 2CaCO3………………………………………(14)
2CaO:V2O5 + 2NaHCO3 ↔ 2NaVO3 + 2CaCO3 + H2O…………………………………………(15)
Leaching in HCl and H2SO4 Solutions
Dicalcium vanadate (2CaO:V2O5) was readily dissolved by either HCl or H2SO4 to form concentrated vanadium solutions. However, HCl solutions of more than 30 grams V2O5 per liter contained too high a concentration of calcium for subsequent precipitation of a high-grade red cake. The H2SO4 solutions containing more than 25 grams V2O5 per liter proved difficult to agitate, filter, and wash because of the large amount of gypsum precipitate. The following leaching procedures were determined to produce solutions from which red cake could be effectively recovered.
The dicalcium vanadate recovered from synthetic solution analyzed 35.5 percent CaO and 55.1 percent V2O5 (CaO:V2O5 mole ratio of 2.1). The dicalcium vanadate was dissolved in HCl solution at about pH 2 by agitating for 0.5 hour at room temperature in a slurry containing 17.1 grams of the salt, 300 ml water, and 17.7 ml concentrated HCl. The solution so formed contained about 30 grams V2O5 per liter. Acid consumption was 4.0 pounds of reagent-grade (C.P.) acid, 37.5 weight-percent HCl, per pound of vanadium.
When employing H2SO4, the dicalcium vanadate was leached at about pH 2 by agitating for 0.5 hour at room temperature in a slurry containing 24 grams of the salt, 514 ml water, and 8.2 ml concentrated, 96 weight-percent, H2SO4. The loss of vanadium with the gypsum precipitate is mainly by coprecipitation, especially when the temperature is allowed to rise above 30° C during acid addition. Standing for any length of time also increases the loss by coprecipitation. The coprecipitation of vanadium with the gypsum was minimized by maintaining the temperature of the slurry below 30° C and by filtering the gypsum precipitate within 0.5 hour after formation. The filtrate contained 25 grams V2O5 per liter. A high vanadium extraction also was obtained by leaching at pH 5; however, the filtrate yielded a red cake more heavily contaminated with calcium. Consumption of concentrated H2SO4 amounted to 2.1 pounds per pound of vanadium.
Leaching in Na2CO3 and NaHCO3 Solutions
The use of Na2C03 and NaHCO3 to dissolve dicalcium vanadate was tried because it is possible to obtain a stronger vanadium solution with these reagents than with acid. Thereby the recovery of vanadium as AMV is made possible. The Na2CO3 and NaHCO3 requirements were based on forming CaCO3 with the calcium present as calcium vanadate. The theoretical quantities of Na2CO3 and NaHCO3 for the 2CaO:V2O5 form of calcium vanadate are 2.1 pounds Na2CO3 and 1.6 pounds NaHCO3 per pound vanadium. Owing to small variations in the CaO:V2O5 mole ratios of the calcium vanadate precipitates processed, the quantities of reagents employed in each test do not exactly correspond to the theoretical values.
The use of Na2CO3 was investigated first. Tests were made to determine the effects of temperature and quality of the Na2CO3 on vanadium extraction in 3- and 6-hour leaches. For a given quantity of calcium vanadate, the volume of leach solution employed was calculated to give a vanadium concentration of 100 grams V2O5 per liter.
A 3-hour leach at 85° to 95° C with 150 percent of theoretical Na2CO3 extracted 99.8 percent of the vanadium, while a 6-hour leach at 25° to 30° C with 125 percent of theoretical Na2CO3 extracted 99.2 percent of the vanadium. Because the final solution had a pH of about 12, which was too high for subsequent recovery of the vanadium as AMV, comparable tests were made in which CO2 gas was bubbled into the solution during leaching. Vanadium extractions of 98.2 and 99.7 percent were obtained with 3-hour leaches using 125 percent of the theoretical Na2CO3 at 25° to 30° C and at 80° to 90° C, respectively.
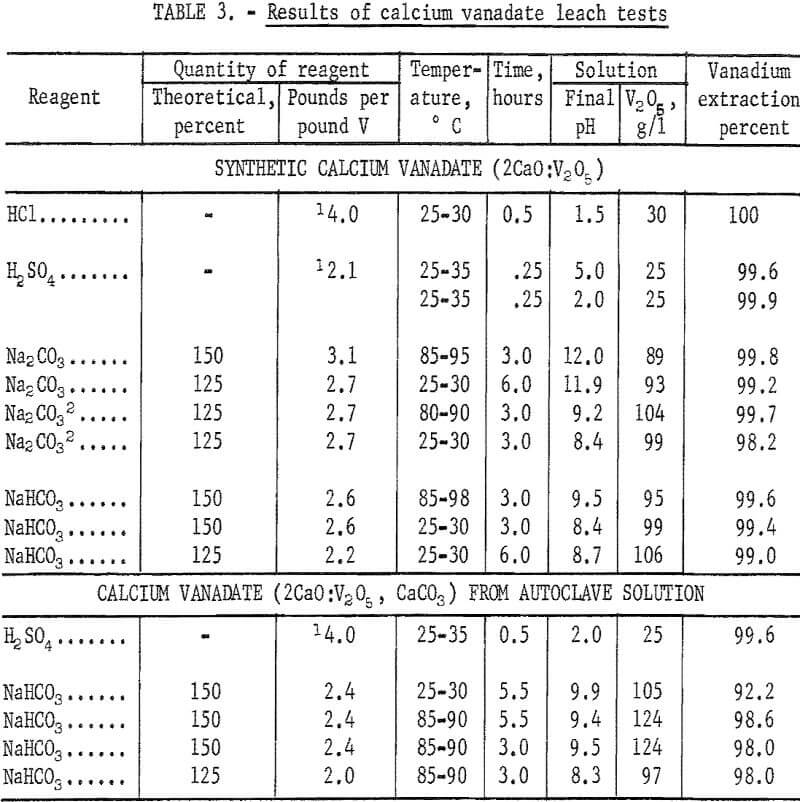
The extraction results obtained with NaHCO3 ranged from 99.0 to 99.6 percent and were comparable to those obtained with Na2CO3. The use of either reagent gave leach solutions ranging in concentration from about 90 to 105 grams V2O5 per liter. Vanadium recovery as AMV obtained from the higher pH Na2CO3 leach solutions was appreciably lower than obtained from the lower pH NaHCO3 or Na2CO3 leach solutions. An apparent disadvantage to leaching in NaHCO3 solution was the evolution of CO2 gas which created a frothing problem particularly when the temperature was increased from 80° to 90° C. Table 3 gives results of the leaching tests made with four different solvents on the dicalcium vanadate recovered from the synthetic solutions.
Dicalcium Vanadate Precipitate Recovered from Autoclave Leach Solution
The dicalcium vanadate-calcium carbonate precipitate recovered from autoclave leach solution, which analyzed 42 percent CaO, 29.5 percent V2O5, and 23 percent carbonate, was leached in H2SO4 and NaHCO3 solutions employing the previously described conditions for processing synthetic calcium vanadate.
When using H2SO4, leaching at pH 2 for 0.5 hour at room temperature dissolved 99.6 percent of the vanadium. Acid consumption was 4.0 pounds of concentrated acid per pound of vanadium.
The use of 150 percent of theoretical NaHCO3 to extract the dicalcium vanadate precipitate and leaching for 5.5 hours at room temperature extracted only 92.2 percent of the vanadium, but leaching at 85° to 90° C under the same conditions increased the extraction to 98.6 percent. The vanadium extraction decreased to 98 percent when leaching at 85° to 90° C for 3 hours employing either 125 or 150 percent of theoretical NaHCO3. Leaching with NaHCO3 gave leach solutions of about pH 9 which ranged in strength from 97 to 124 grams V2O5 per liter. The results of leaching the dicalcium vanadate precipitate recovered from autoclave solution were, therefore, in close agreement with the results obtained by processing synthetic dicalcium vanadate. Test results are given in table 3.
Vanadium Product Recovery
The reactions for precipitating vanadium products from the acid and alkaline extracts of the dicalcium vanadate are represented by the following equations:
H4V6O17 ↔ 3V2O5………………………………………………………………………………….(16)
2H4V6O17 + CaCl2 ↔ CaH2V6O17·3V2O5 + 2HCl + 2H2O…………………………(17)
H4V6O17 + 2NaCl ↔ Na2H2V6O17 + 2HCl……………………………………………….(18)
H4V6O17 + 2NH4Cl ↔ (NH4)2H2V6O17 + 2HCl……………………………………….(19)
6NaVO3 + 2H2SO4 ↔ Na2H2V6O17 + 2Na2SO4 + H2O……………………………(20)
6Na3VO4 + 8H2SO4 ↔ Na2H2V6O17 + 8Na2SO4 + 7H2O………………………..(21)
6NaVO3 + 2H2SO4 + 2NH4Cl ↔ (NH4)2H2V6O17 + 2Na2SO4 + 2NaCl + H2O…………(22)
NaVO3 + NH4Cl ↔ NH4VO3 + NaCl……………………………………………………………………….(23)
The products contained a variable quantity of combined water that is not shown in the equations.
The specifications for red cake were reported previously. The specifications for AMV vary depending upon the intended use. The preparation of high-purity AMV is covered by Vezina and Gow.
Vanadium is generally recovered from alkaline vanadate solutions by acidifying with H2SO4 to pH 2 to 3 and heating the agitated solution to near boiling to precipitate the commercial product known as red cake. The formula for red cake is usually given as sodium hexavanadate (Na2H2V6O17), but red cake products vary considerably from this formula depending on the pH of precipitation and the cations present in solution. When the red cake is precipitated in the presence of an ammonium salt, (NH4)2H2V6O17 (ammonium hexavanadate) may be formed, but generally a mixed ammonium-sodium hexavanadate salt is precipitated. Usually, the stronger acid solutions yield red cake products of lowest sodium content, and highest loss on ignition, probably because of an increase in polyacid content of the red cake. Red cake may be dissolved in soda ash solution to obtain an enriched alkaline sodium vanadate solution from which AMV is crystallized by adding NH4Cl or (NH4)2SO4 and agitating at room temperature.
Tests were made to recover red cake or ammonium hexavanadate products from the acid extracts and AMV from the alkaline extracts of the dicalcium vanadate recovered from synthetic sodium vanadate solution. Similarly, red cake and AMV products were recovered from the H2SO4 and NaHCO3 extracts of the dicalcium vanadate precipitate recovered from the autoclave leach solutions.
HCl Extract
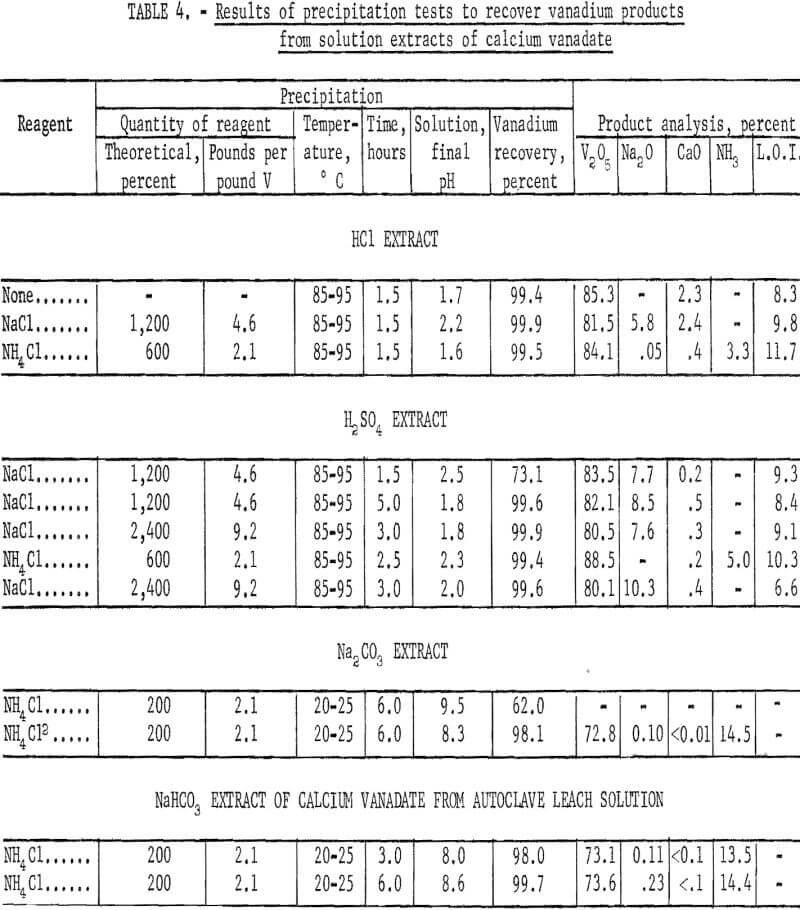
The HCl extract of dicalcium vanadate recovered from synthetic solution, containing 30 grams V2O5 per liter, was agitated and heated for 1.5 hours at 85° to 95° C to precipitate a red-cake-type product. The pH of the solution was 1.5 before heating and 1.7 after precipitation. Vanadium recovery was 99.4 percent in a product that analyzed 85.3 percent V2O5, 2.3 percent CaO, and 8.3 percent loss on ignition. The analysis indicates that a mixed calcium hexavanadate-polyvanadic acid salt was formed by precipitation in the absence of sodium ions. A similar test was made with the objective of precipitating a sodium hexavanadate-red cake by adding enough NaCl to the HCl extract to provide 12 times the theoretical requirement of sodium in red cake (Na2H2V6O17). The pH after agitation for 0.5 hour at room temperature was 1.6, and the final pH after precipitation for 1.5 hours at 85° to 95° C was 2.2. Vanadium recovery was 99.9 percent in a red-cake type, product that analyzed 81.5 percent V2O5, 5.8 percent Na2O, 2.4 percent CaO, and 9.8 percent loss on ignition.
In an attempt to avoid lime contamination a test was made precipitating in the presence of six times the theoretical NH4Cl, based on forming (NH4)2H2V6O17. The final pH was 1.6. Recovery of vanadium was 99.5 percent in a product that analyzed 84.1 percent V2O5, 0.05 percent Na2O, 0.4 percent CaO, 3.3 percent NH3, and 11.7 percent loss on ignition. A longer reaction time and adjustment of the pH closer to 2.0 probably would result in more complete conversion to ammonium hexavanadate and a better elimination of lime.
H2SO4 Extracts
The vanadium recovery was increased from 73.1 to 99.6 percent by increasing the reaction time from 1.5 to 5.0 hours when adding 12 times the theoretical NaCl, based on forming Na2H2V6O17, to the H2SO4 extract and heating to 85° to 95° C. By doubling the amount of NaCl added, virtually complete recovery of vanadium was obtained in a reaction time of 3 hours. The red-cake-type products assayed 80.5 to 83.5 percent V2O5, 7.6 to 8.5 percent Na2O, 0.2 to 0.5 percent CaO, and 8.4 to 9.3 percent loss on ignition. A higher grade product was obtained by adding six times the theoretical NH4Cl, based on forming (NH4)2H3V6O17, to the acid extract and reacting for 2.5 hours. Vanadium recovery was 99.4 percent in a product that analyzed 87.5 percent V2O5, 0.2 percent CaO, 5.0 percent NH3, and 9.8 percent loss on ignition. A complete conversion to ammonium hexavanadate was not attained.
The H2SO4 extract of the dicalcium vanadate precipitate recovered from leach solution was processed by adding 24 times the theoretical NaCl, based on forming Na2H2V6O17, and by heating at 85° to 95° C for 3 hours. The NaCl added amounted to 9.2 pounds per pound of vanadium. Vanadium recovery was 99.6 percent in a red-cake-type product that analyzed 80.1 percent V2O5, 10.3 percent Na2O, 0.4 percent CaO, and 6.6 percent loss on ignition. Similar results were obtained using the same procedure on the synthetic solution. The test results are given in table 4.
Na2CO3 and NaHCO3 Extracts
The pregnant Na2CO3 leach solutions of about pH 12 were processed to recover AMV by adding 200 percent of the theoretical NH4Cl, based on forming NH4VO3 and agitating at room temperature for 6 hours. The addition of NH4Cl lowered the pH from 12 to 9.5; however, at this pH the vanadium recovery was only 62 percent. By using H2SO4 to decrease the pH from 9.5 to 8.6, prior to adding the NH4Cl, the vanadium recovery was increased to 98.1 percent in a product that analyzed 72.8 percent V2O5, 0.10 percent Na2O, <0.01 percent CaO, and 14.5 percent NH3. As an alternative to using H2SO4 for decreasing the pH, CO2 gas was bubbled into the solution during or after leaching to lower the pH from 12 to 8.7. A product similar to the latter was obtained after processing with NH4Cl at room temperature. The NaHCO3 extracts of synthetic dicalcium vanadate as stated previously, were within the desired pH range of about 8.7, and recovery of AMV presented no problems.
The NaHCO3 extract of the dicalcium vanadate precipitate recovered from leach solution was processed by adding 200 percent of theoretical NH4Cl and agitating at room temperature. By increasing the agitation time from 3 to 6 hours, the vanadium recovery in a fairly typical AMV product was increased from 98.0 to 99.7 percent. Consumption of NH4Cl was 2.1 pounds per pound of vanadium. The test results are given in table 4.
Conclusions
Calcium vanadates corresponding to 2CaO:V2O5, 3CaO:V2O5, and 4CaO:V2O5 were precipitated from alkaline sodium vanadate solutions. The precipitation of a specific calcium vanadate was dependent on the pH of the solution and quantity of available calcium present. The use of calcium chloride and caustic soda gave best results. Emphasis in the present investigation was given to the precipitation of dicalcium vanadate since this form of calcium vanadate was the most economical to precipitate and process. From the investigation of the precipitation and processing of dicalcium vanadate recovered from synthetic sodium vanadate solutions and an autoclave leach solution, the following conclusions were drawn;
- The precipitation of dicalcium vanadate (2CaO:V2O5) is a simple method for recovering vanadium from a low-tenor alkaline leach solution (less than 10 grams V2O5 per liter). Vanadium recoveries ranged from 98 to 99.5 percent when 110 to 130 percent of theoretical CaCl2 was employed. Reagent consumption was 2.4 to 2.8 pounds of CaCl2 and 0.5 pound of NaOH per pound of vanadium.
- Dicalcium vanadate is readily extracted in HCl, H2SO4, Na2CO3, and NaHCO3 solutions to obtain vanadium solutions containing from 25 to 125 grams V2O5 per liter. Vanadium extractions ranged from 98 to 100 percent; however, less lime contamination of the final vanadium products resulted when H2SO4 and NaHCO3 were used as leaching reagents. Consumption of H2SO4 ranged from 2.1 to 4.0 pounds per pound of vanadium and consumption of NaHCO3 was 2.4 pounds per pound of vanadium. Mixing and filtering were necessary with H2SO4, whereas mixing, heating, and filtering were required with NaHCO3.
- Processing the extracts of dicalcium vanadate resulted in high recoveries of marketable grades of either red cake or ammonium metavanadate. A vanadium recovery of 99.6 percent was obtained in red cake precipitated from the H2SO4 extract, and a recovery of 99.7 percent in ammonium metavanadate from the NaHCO3 extract. The red cake contained 0.4 percent CaO and the AMV less than 0.1 percent CaO as the principal impurities. Reagent consumption was 9.2 pounds NaCl per pound of vanadium for the acid extract and 2.1 pounds NH4Cl per pound of vanadium for the NaHCO3 extract. Conventional unit operations were employed in the processing to recover the alternative red cake or AMV products.
