Table of Contents
Although the bromo-cyanide or Diehl process for the extraction of gold from its ores is used on several of the Kalgoorlie mines, very little information has been published on the original process, and no details are available from the various mines now using it.
The following description of the process as carried on at the Hannan’s Star plant, where several modifications were introduced by the author, will therefore be of interest to mining men, and it is hoped that some discussion on this paper will provide further useful information to the mining community.
The process was first installed on the Hannan’s Star mine, where a full working plant was erected by the London-Hamburg Co. In their contract they guaranteed a 91 per cent, extraction on a sulphide ore of 15 dwt. value. This was considered a very high extraction at the time, and the plant successfully carried out its work.
The new features in the process were the finer grinding of the ore, for which purpose the tube mill was introduced, and the addition of a solution of bromo-cyanide to the vats under ordinary cyanide treatment, whereby a higher extraction was obtained in a shorter time. The main difficulty in the process was the easy manufacture of the bromo-cyanide solution, but this was eventually overcome. The mixed salts now supplied by the London-Hamburg Co. are in every way satisfactory, and are supplied at such a price that the process compares favourably with any other from a cost point of view.
Most of the Hannan’s Star ore which has been treated by the process was of low grade, and hence the percentage extraction shows low, but the average value of residues for 1904 and 1905 were 1 dwt. 2 grs. and 1 dwt. respectively on ore averaging 10 dwts. 7 grs. and 8 dwt. 9 grs. respectively,. which must be considered good work. On high grade ore the process was equally successful. Some 15,000 tons from the Brown Hill Extended mine, averaging about 4 ozs. gold per ton, and containing telluride, were treated by Mr. O. B,. Ward in 1903 for an average extraction of 95 per cent. On Hannan’s Star ore the average cost for bromo-cyanide, extending over a period of twelve months (September, 1904, to October, 1905), was 9.75 pence per ton of ore treated, and the extraction was entirely satisfactory. On the same class of ore (sulphide), roasting would cost about 2s. 9d. per ton, so that we have a balance of 2s. per ton in favour of the chemical, and the saving of very heavy first cost in furnace plant.
In other places, where fuel and furnace supplies were expensive, the chemical process would show a much more decided advantage.
The process requires more metallurgical skill and constant attention to the progress of each vat under treatment; but, if this is available, the results are highly satisfactory, and the process has very definite claims as a cheap and efficient method of treatment for such ores as the Kalgoorlie sulpho-tellurides.
The author took charge of the Hannah’s Star plant in July, 1904, and the process at that time was as follows:
The ore from the rock-breaker was dry crushed in two No. 5 Krupp ball mills, with steel wire screens of 27 holes per lin. inch. From the mills it passed to a mixer, over amalgamated plates, and then to a classifier at the end of the tube mill. Here a separation was made, the overflow going to the slimes pumps, and the underflow into the tube mill. The discharge from the mill was elevated back to the classifier, where separation of the insufficiently ground portion was again effected. The slimes pumps delivered to two sets of spitz-luten, the underflows from which passed back to the tube mill, and the overflows to the pointed settling boxes, where the pulp was thickened up before passing to the agitator vats. The point aimed at was to slime the whole of the ore to pass a sieve of 150 holes per lin. inch. In practice about 5 per cent, would remain on the “ 150 ” sieve, but the whole of it would pass the “ 100 ” sieve.
There were four agitator vats of 50 tons dry ore capacity each. These each took on an average 16 hours to fill, representing 75 tons per day through the ball mills. Their tonnage was determined, for working purposes, by weighing the contents of a bottle, filled to its containing mark by dipping into the vat, and by measuring the volume of pulp in the vat. From the volume, tonnage, and specific gravity, the dry weight of ore was obtained.
A mechanical sampler was arranged between the ball mills and the mixer, which cut out a definite quantity periodically. This bulk sample was “ quartered ” down, which gave the ore sample for the day. A vat when full was given its charge of KCy, and three hours afterwards a “ dip ” KCy residue was taken, and the charge of BrCy solution added. After a total agitation of 20 hours a quantity of lime was added and the vat “ pressed.” The quantity of BrCy added was varied according to the residues of preceding vats and the value of the ore being treated as shown by the daily ore sample.
Every sixth frame in a filter-press was sampled, and the bulk sample from the whole vat quartered down to represent the final residue of that vat. The KCy residue was washed on a small vacuum filter and assayed, while the final residues were assayed after drying. From the daily ore samples KCy and final residues, the total extraction and extraction by plain KCy and by BrCy were determined.
Bromo-Cyanide Solution
The bromo-cyanide solution is made according to the following equation:
2 KBr + KBrO3 + 3 KCy + 3 H2SO4 = 3 BrCy + 3 KaSO4 + 3 H2O.
And its action in the treatment vat is supposed to be as follows:
BrCy + 3 KCy + 2 Au = 2 K AuCy2 + KBr.
The first two quantities in equation 1 are contained in the mixed salts supplied by the London-Hamburg Co., and which have about the following composition :—
40-44 % of Br as KBr
20-22 % of Br as KBrO3;
the proportion of Br as bromide being about twice that of Br as bromate. A 30-lb. charge is usually made up, and for this the following weights are taken :—
H2SO4………………………….50 lbs.
KCy……………………….20 lbs.
Mixed salts………………..36.8 lbs.
The KCy being 93 per cent., and the H2SO4 63 per cent, (chamber acid). The solution is made in a closed wooden vessel, stirred by rotating arms, and holding about 200 gallons. In making up a charge, portion of the water and all the H2SO4 are first mixed, and allowed to cool to normal temperature. The KCy, which is dissolved in a separate vessel in sufficient water to fill the mixing vessel, is then run in, and at the same time the proper weight of “ mixed salts ” gradually added. The whole is then agitated for six hours before being used, and in a closed vessel will retain its strength for some days.
The cost of a 30-lb. charge of BrCy is about £4 10s., made up as follows:
50 lbs. H2SO4 at 2d., 20 lbs. KCy at 8.37d., 36.8 lbs. salts at 1s. 10d.
From each charge mixed, a dip sample is taken and tested with a standard Na2S2O3 solution, using potassium iodide as an indicator.
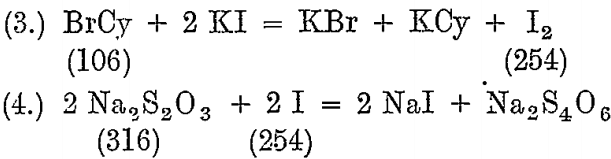
The standard solution is made so that 1 c.c. = .02 BrCy, and for this about 93.6 grams of ordinary photographic crystals, Na2S2O3 5 H2O, are dissolved in 1 litre of water.
A solution of copper sulphate is used for standardizing the above.
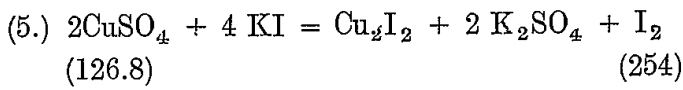
126.8 Cu liberates 254 I, so that it corresponds to 106 BrCy and 496 Na2S2O3 5 H2O.
The solution is made by dissolving one gram of pure copper foil in acid, converting to sulphate, and dissolving in 100 c.c. water.
10 c.c. contains .1 grain Cu = .0836 BrCy;
then 1 c.c. hypo solution = .02 gram BrCy.,
and 4.18 hypo solution = .0836 gram BrCy.
In testing BrCy solutions, 5 c.c. are usually taken, Na2CO3 solution added till alkaline, and then acetic acid till acid. A few crystals of KI and some starch solution are then added, and the whole titrated with the standard Na2S2O3 solution.
Example :— If 5 c.c. BrCy solution took 3.2 c.c. hypo then
3.2 x .0836/4.18 x 20 = 1.28%
This method of testing BrCy solutions is different to that used on other mines, where they titrate direct with Na2S2O3 without first neutralizing the H2SO4. The following is a series of tests made on a number of 30-lb. BrCy solutions by direct method, A, and, after neutralizing, B :—

These show that by direct titration the test is usually low, and also that it gives irregular results, depending on the amount of free H2SO4 present. There seems to have been some doubt as to whether a BrCy solution increases in strength after, say, one hour’s agitation, but the following tests, which were made, show that it does increase up to about 8 hours.
After 1 hour mixing solution tested 0.56 % BrCy.
After 2 hour mixing solution tested 0.72 % BrCy.
After 3 hour mixing solution tested 0.72 % BrCy.
After 4 hour mixing solution tested 0.80 % BrCy.
After 5 hour mixing solution tested 0.80 % BrCy.
After 6 hour mixing solution tested 0.88 % BrCy.
After 7 hour mixing solution tested 0.92 % BrCy.
After 8 hour mixing solution tested 1.00 % BrCy.
After 24 hour mixing solution tested 1.04 % BrCy.
At the time the author took charge it was generally known that if “ plant ” solution became too alkaline, either through a change in the ores, or the addition of too much lime either to the ore before crushing or after the bromo-cyanide treatment, that the extraction by BrCy fell considerably, and for this reason an occasional test of the plant solution was made for alkalinity, but not till several high residues had occurred.
Under the old system, too, the KCy did not have sufficient time by itself; the values of the ore and KCy residues of each vat were not known, and the amount of BrCy that should be added was more or less a guess. When it is remembered that every 5 lbs. of BrCy added to a 50-ton vat represents a cost of 4d. per ton of ore, that the action of the BrCy can be made just as effective after long KCy treatment, and that any excess of BrCy is of no advantage, it will be seen how important is that the value of the KCy residue should be known after sufficient agitation (say 12 hours), and the condition of the vat tested as to alkalinity before adding the BrCy. In any case the action of the BrCy is of short duration, not exceeding 4 hours, so that if 20 hours’ total agitation can be allowed, it is better to give 16 hours with KCy and then add the BrCy. It is preferable, however, to keep the vat under KCy treatment until the KCy residue is known, then correct the alkalinity and add the BrCy. This could easily be done with extra vat capacity. Total alkalinities are determined with standard solutions of HCl and NaHO, using phenolthalien as indicator. The following experiments were carried out to determine what was the most suitable degree of alkalinity for bromo-cyaniding.
Six sludge samples were taken at the same time from a vat which had KCy treatment. The solution was filtered off one, and the quantity of a weak H2SO4 solution required to about neutralize it was determined. It took 40 c.c., so that to the sludge samples was added the following quantities of H2SO4 solution respectively :—
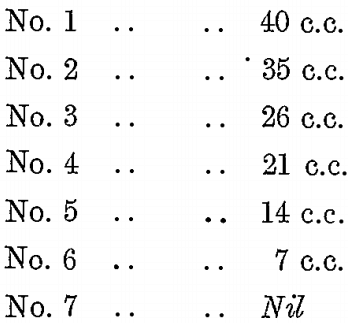
To each sample was also added 7 c.c. of BrCy solution, and the bottles were sealed and agitated for 8 hours. The solutions were then poured off and tested for alkalinity, while the residues were washed and assayed. The following were the results :—
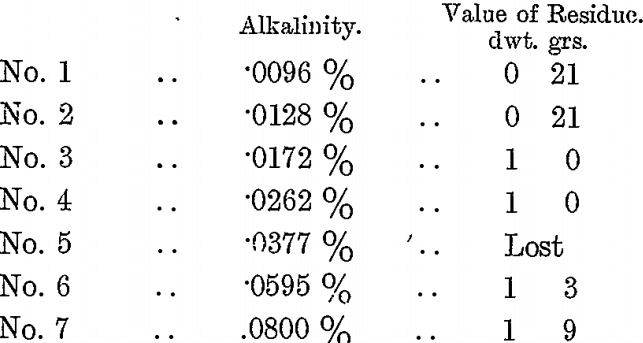
The KCy residue of the vat was 2 dwts., the final “ press ” residue 1 dwt. 9 grs., and the alkalinity of the solution from the KCy residue was .08 per cent., and these are included as No. 7 in the above table. From these tests it will be seen that as the alkalinity was reduced so the residues fell in value in almost exact proportion; they prove conclusively that BrCy does not act well in a too alkaline solution, and that the best action is obtained when the alkalinity is about .01 per cent, to nearly neutral.
A second series of tests on a different value ore (about 14 dwts.) was made; as in the previous case varying quantities of H2SO4 and 7 c.c. of BrCy were added to the bottles, agitated for 12 hours, and the residues washed and assayed.
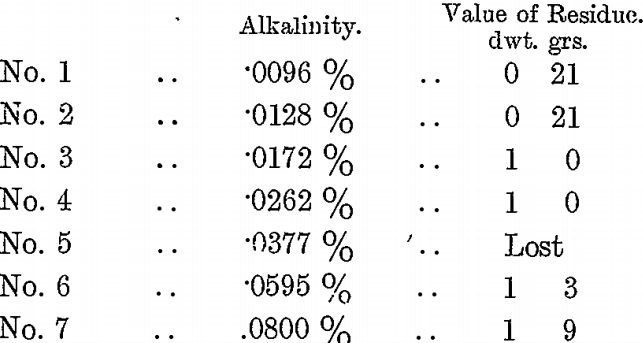
The KCy residue of this vat was 4 dwts. 12 grs., and the final press residue 1 dwt. 9 grs. It became the practice, therefore, to have the alkalinity of the vats during KCy treatment at between .02 and .03 per cent., to keep the vat agitated till the value of the KCy residue was known, then correct the alkalinity to about .01 per cent. or less by H2SO4 and add BrCy according to the value of the KCy residue. A better extraction was thus obtained at less cost for BrCy, at the expense of a small amount of H2SO4 and extra agitation.
Owing to an insufficient number of vats, they could not always be kept back for the KCy residue, but on every occasion on which it was done a certainty could be made of the residue being low and the consumption of BrCy a minimum. A number of experiments were carried out to explain this reduced action in an alkaline solution, but nothing definite was proved, although it is possibly due to the presence of KHO.
If a solution of KHO be added to BrCy it is almost immediately destroyed. No smell of BrCy is left, and no test can be obtained with Na2S2O3.
(6.) BrCy + 2 KHO = KBr + KCyO + H2O.
Action of BrCy on FeS2 and metallic Fe.-Various experiments were carried out to determine the action of BrCy on FeS2 and finely divided metallic Fe.
The FeS2 used was panned off from finely crushed ore, while metallic Fe was picked by a magnet from the sludge in a vat. Freshly prepared iron filings were also tried. Small quantities of these were agitated with a previously tested BrCy solution. Action at once began, and in a short time the BrCy was destroyed. All smell of BrCy disappeared, and no test could be obtained with Na2S2O3. The result is a greenish-blue solution of a ferrous salt, from which a dark green ferrous salt is precipitated by NH4OH.
These experiments showed that both FeS2 and metallic Fe are bromo-cyanicides, that finely-divided metallic Fe is the more active, and that there is sufficient of the latter produced in grinding the ore to destroy the amount of BrCy usually added to a vat.
The BrCy seems to act more, by breaking up the FeS2 than by actual solution of the gold, but it is probable that a great portion of it is destroyed by the finely-divided metallic Fe, without, of course, any beneficial result as far as setting free or dissolving gold is concerned.
Effect of adding Lime
Experiments were carried out to show the effect of adding ordinary commercial lime to vats after BrCy treatment, and it was found that a re-precipitation of gold took place to a small extent. To show this in a magnified way, two samples of solutions—A and B—were taken from a vat after BrCy treatment. An excess of lime was added to A, agitated for a few hours, and then assayed, with the following results :—
A solution assayed 3 dwts. 21 grs. per ton;
B solution assayed 5 dwts. 5 grs. per ton;
showing that the lime precipitated 1 dwt. 8 grs. of gold per ton from solution, or 25 per cent, of its contents.
This is probably due to the presence of carbon or occluded gases, such as hydrogen.
The results of these experiments have suggested certain improvements in bromo-cyaniding, some of which the author was able to adopt, and the others are recommended. They may be summed up as follows:—
- The daily ore sample should be taken in the morning, and assayed as soon as possible, so as to know what was the value of the ore passing to the vats in the previous 24 hours.
- The pulp should have a long KCy treatment.
- A vat should be kept under KCy treatment till the value of the KCy residue is known.
- The alkalinity of the vat should then be determined and corrected to .01 per cent, by H2SO4 before adding BrCy.
- The quantity of BrCy added should then be determined from the value of the KCy residue, the onnage of the vat, &c.
- The lime added to the ore during crushing should be varied according to the alkalinity test after KCy treatment, so that the plant solution tests about .02 per cent.
- Lime water should be made and added to the vats or to the solution from the presses, instead of adding lime to the vats.
- All metallic iron should be removed in some way from the ore before treatment, as it is both a cyanicide and bromo-cyanicide.
