
In order to get a preliminary view of the question of gold absorption in cupelling gold bullion, certain samples of very pure gold, which were being sent out to various laboratories in the Mint service for comparative assaying, mere assayed in sets of sis with three proofs. The nine ctipe!i were arranged in the muffle in a square, the three proofs occupying the middle line from front to back. On completion of the assays, the used cupels were properly marked to allow their position in the muffle to the Bureau laboratory accompanied by a report giving the, assay results obtained in each Bone-Ash Cupels, including the proof figures.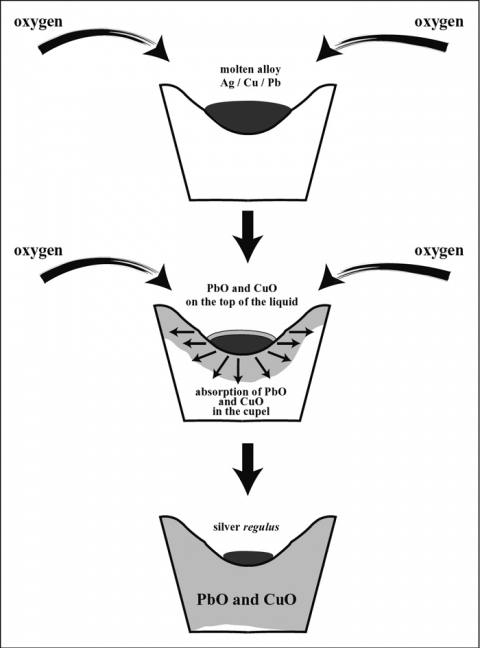

Bone-Ash cupels have been used from time immemorial to absorb litharge, and accompanying oxides, in assaying. Doubtless, also, from the earliest days cupels have been most unjustly blamed for much poor assaying. It has long been known that the precious metals go with the litharge into the cupel. This occasions a loss of precious metals and reduces the results of the assay. There have been many investigations of cupels and the most diverse views have been expressed, the extremes being the statements of Lodge that every cupel has some effect upon the loss in assaying telluride ores and of the Campredons that coarse and fine and hard and soft cupels all give the same loss of silver when cupelling the same weights. The truth lies between these statements and much nearer the latter. In a vast majority of cases the influence of the cupel is not dominant and is outweighed by other conditions, particularly the temperature of the cupelling bead.
At the Spokane meeting of the Institute I presented a paper on the Assay and Valuation of Gold Bullion, which recorded the results of many hundreds of assays made in the Mint service under everyday commercial conditions, which of necessity differ from scientific conditions.
In following this matter up, for the purpose of increasing the accuracy of our commercial methods of bullion assays, the question of the absorption of gold by the cupel was found to be a most important one and was taken up for investigation. In a lesser degree, the silver absorption is important and interesting, but at present it appears to be far more complicated and, while many figures showing silver absorption may be found through this paper, much further investigation is required to put the subject upon a satisfactory basis.
In order to get a preliminary view of the question of gold absorption in cupelling gold bullion, certain samples of very pure gold, which were being sent out to various laboratories in the Mint service for comparative assaying, were assayed in sets of six with three proofs. The nine cupels were arranged in the muffle in a square, the three proofs occupying the middle line from front to back. On completion of the assays, the used cupels were properly marked to show their position in the muffle and forwarded to the Bureau laboratory accompanied by a report giving the assay results obtained in each cupel, including the proof figures.
In the Bureau laboratory, the used portion of each cupel was carefully separated from the unused portion and ground to pass 100-mesh. The powder was weighed and mixed with an equal weight of litharge and of bicarbonate of soda and 0.5 gram of flour. Owing to the refractory nature of the phosphate of lime, a small amount of cryolite was added to liquefy the fusion. The proportion of bone ash saturated by the litharge varied somewhat in the different laboratories, but in general one-eighth to one-sixth the weight of the used cupel of cryolite would give a very satisfactory fusion, when melted at a slightly high temperature.
The resulting lead buttons were carefully cupelled with feather litharge, the final bead being weighed and then parted for gold. The general rule followed in preparing the cupels for assay was to be sure and include all the stained portion. In doing this, more or less unstained bone ash had to be taken. A large amount of the unstained ash required the larger amount of cryolite. In later work with heavier cupels, more flour was required.
In this preliminary test, six sets of nine cupels (54) from each one of five laboratories were assayed in the Bureau laboratory. This made a total of 270 cupels, but for various reasons eight assays were lost, leaving a total of 262.
The details of the method of assaying followed in each laboratory are briefly summarized in Table 1.
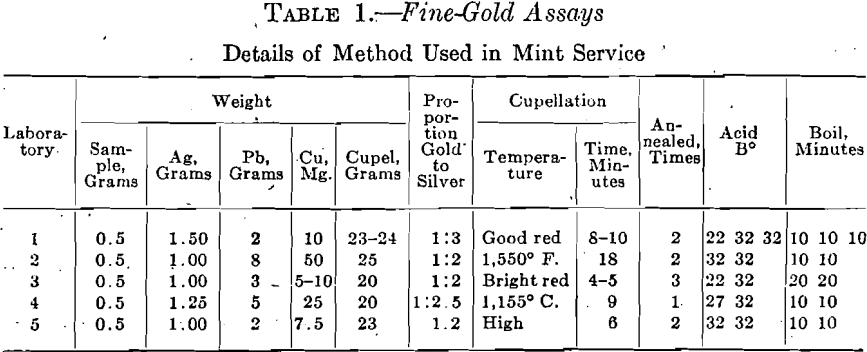
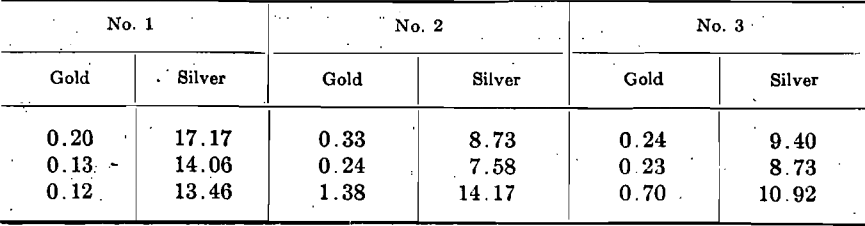
The amount of gold absorbed at the laboratories No. 1, 2 and 3 varies widely, but shows a considerable agreement amongst themselves. Laboratory No. 4 shows very large and widely varying absorptions of gold, while the absorptions at laboratory No. 5 are very small and agree very closely. These results are shown in Table 2.
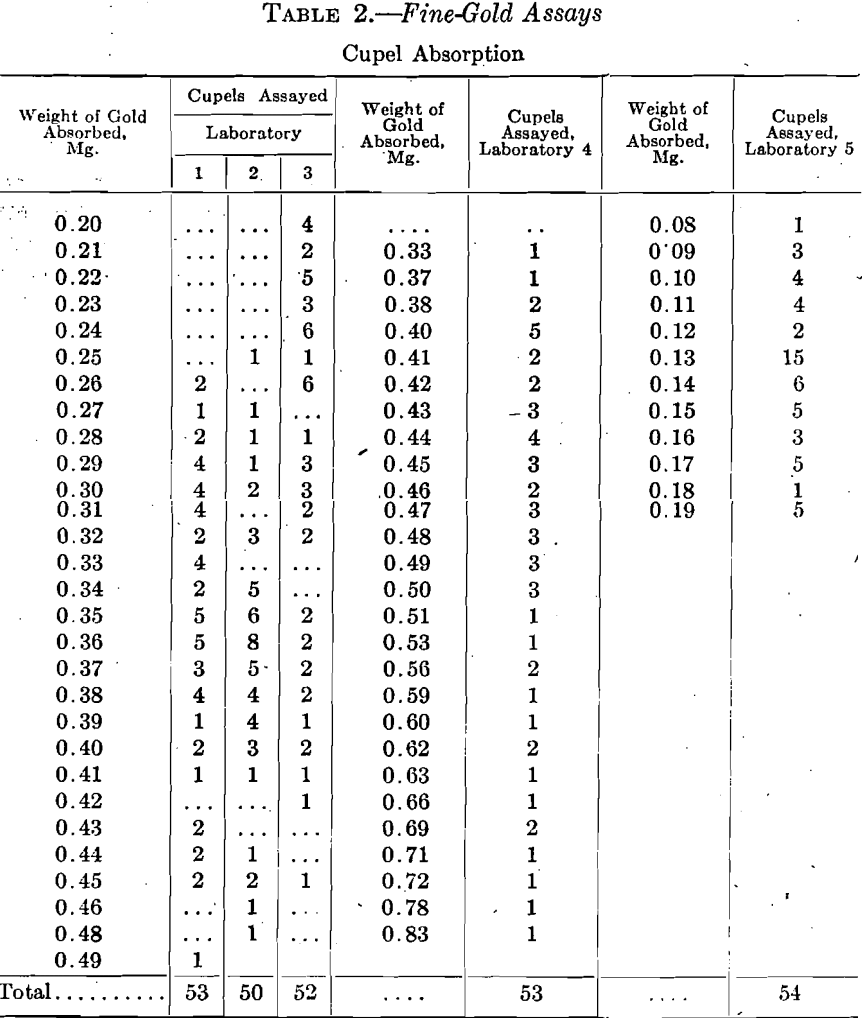
The amount of silver absorbed by the cupels shows an astonishing variation and for the present simply the highest and lowest amounts in each set are given, in Table 3.
This set of cupel assays was made for the purpose of getting a general preliminary view of the field. The 270 cupels may be taken as typical
of the general practice of cupel making in the Mint service at that time. Each one had been taken from a lot that had passed all the usual visual-examination and other tests. Each one had successfully passed the test of actual use, and was therefore entitled to be called a good cupel. Consequently, the determination of the amount of gold absorbed by each cupel should give a set of typical figures.
It was fully appreciated that the cupel absorption was influenced by a variety of circumstances and that some differences could naturally be looked for, but such wide variations as the table shows were entirely unexpected. It is possible, but hardly probable, that some of the cupels contained beads. Certainly the presence of beads could not account for the variations.
The next step in the investigation was to gather a set of samples of bone ash from the various laboratories and subject them to a screen analysis. As in the case of the cupels, these samples may be taken as typical, as they present five varieties of bone ash that had been accepted by five institutions as suitable for making cupels and had been used for that purpose. It would have been better if we could have tested the actual bone ash used in making the cupels assayed, but it was then too late for that and these screen analyses simply give a general idea of the practice at each laboratory.
Table 4 gives these screen analyses, together with the screen analysis of the bone ash at that time in use in the Bureau laboratory. This ash
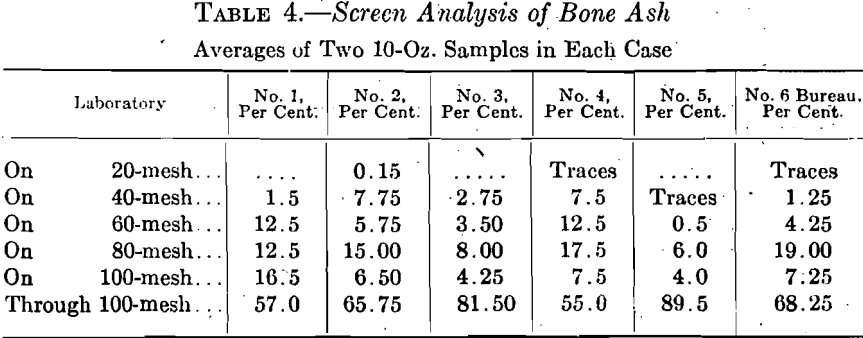
had been obtained from laboratory No. 1. It is therefore shown that considerable variation might occur in the screen analysis of the ash as used at a single laboratory at different times.
In making screen analyses of bone ash, it is absolutely essential that the samples be thoroughly dry. This requires prolonged exposure to a gentle heat, preferably a little above 100° C.
In order to further test the question, this table of screen analyses was submitted to the five laboratories without specifying the sources of the bone ash or informing them of the results of assaying the cupels, and each one was asked to select the best ash in their judgment for making cupels. Laboratory No. 1 declined to commit itself; No. 2 selected ash No. 4 which had given the worst results in the assay tests; No. 4 selected ash No. 1, and Nos. 3 and 5 selected both No. 3 and No. 5 ash.
Each laboratory supplied a full and complete description of its methods of securing what was considered a proper bone ash and of manufacturing the cupels. The tests applied to the bone ash had been crude and empirical. One assayer said: “The best test we can make with new ash is the practical working, that is making a large number of assays (proofs), making a careful examination of the cupels and of the resulting assays.” Another said: “The tests applied to the bone ash when purchasing have been simply to specify the grade, and when it has been received to see that it is clean, being free from dirt, and passing it through the hand to see if it feels all right, and if it will make a firm smooth cupel. The cupels after drying are tried out in the muffle to see if they absorb the lead and do not check or crack, and if they hold up the bone ash is accepted.” A third assayer sifted the ash through 60-mesh and if more than 4 per cent, remained rejected the sample. A fourth assayer said: “I apply no special tests to bone ash beyond inspecting and handling it. If it does not make a satisfactory cupel I reject it.”
The method of making cupels is very simple and substantially the same at each institution. The bone ash is moistened, generally with water only, until it will cohere into a lump on gentle pressure in the hand. After being allowed to stand, it is fed into a mold, a plunger is applied to form the cupel, and the finished cupel is removed from the mold. Different styles of machines, both hand and power, are used to form the cupel and different pressures are applied. The size, shape, and weight of the cupel vary, but, owing to the wide variation shown in the amount of gold absorbed when the cupels were used, it scarcely seemed worth while to go into a discussion of these details at this stage of the investigation, although they were of considerable value in carrying on the work.
Owing to the very large button of gold and silver obtained in assaying gold bullion, the question of the smoothness of the surface of the cupel is not quite so important as it is in the assaying of ores where minute buttons are obtained, but the surface must be fairly smooth.
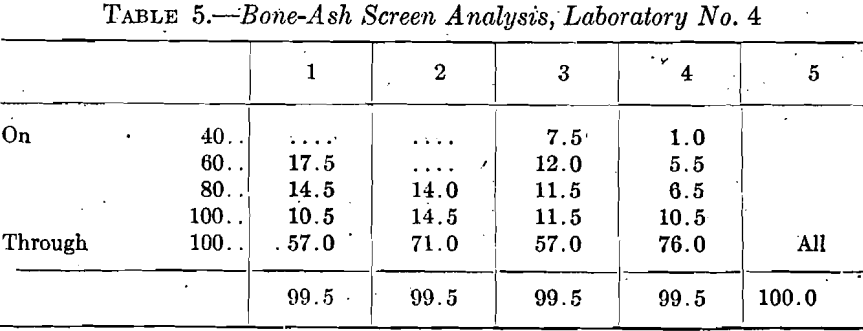
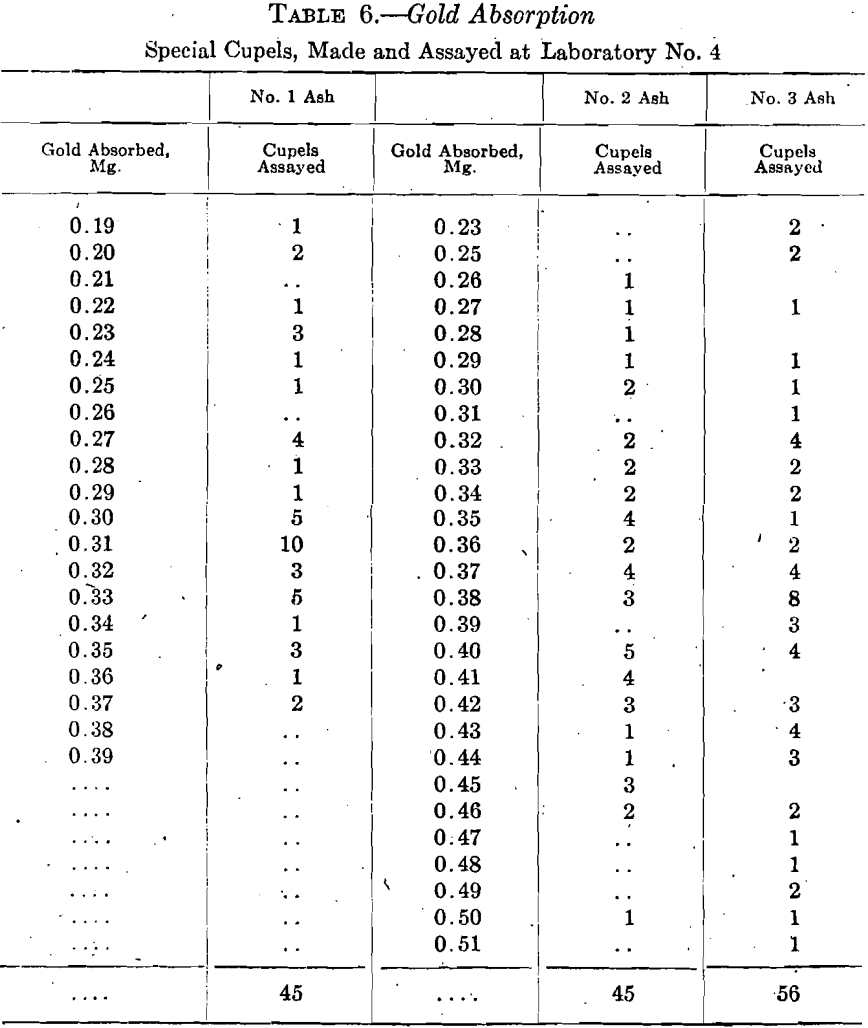
A casual inspection of the cupel absorption table suggests that the cupels made in laboratory No. 4 were unreliable and in need of improvement. New cupels were therefore made from five different samples of bone ash shown by the screen analyses in Table 5.
Cupels made from Nos. 1 and 2 ash, 45 of each, and 56 cupels made from No. 3 ash, were used in assaying very fine gold, 0.5 gram, and the used cupels were assayed for gold and silver. Table 6 summarizes the gold figures obtained.
While these gold absorptions agree better than the first set from No. 4 laboratory, yet most of them are much too high.
Cupels Nos. 1 and 2 were used in sets of nine each, No. 3 in sets of nine and ten, the proportion of silver to gold being 2½:1. Table 7 shows the highest and lowest silver absorption found in each set.
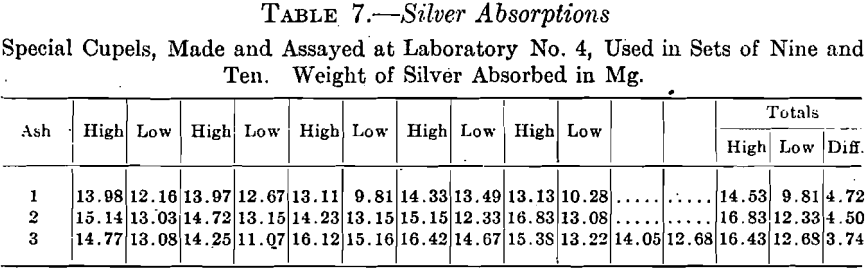
Cupels made from ash No. 4 were tested in assaying standard coin gold, 900 gold and 100 copper with a trifle of the copper replaced by silver, 0.5 gram being used for the assay. They were tested in 12 sets of ten each under varying conditions. At this time all the absorption results will be grouped together, leaving the discussion of the effect of the varying group conditions until later.
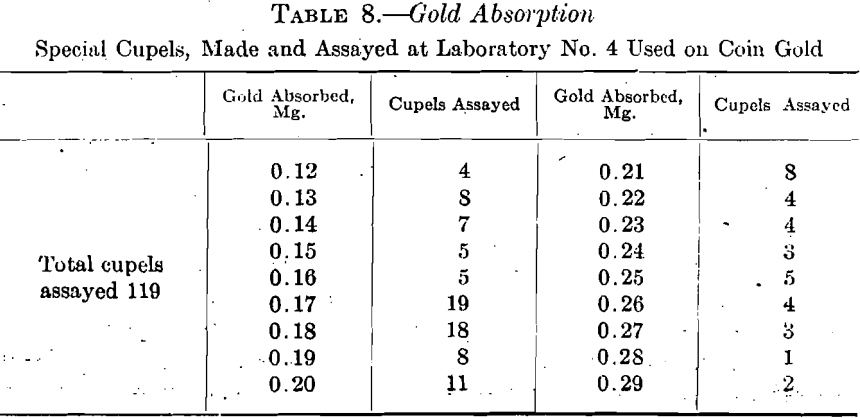
Table 9 shows the highest and lowest silver absorption found in each set of ten cupels. The proportion of silver to gold in the coin assays was 2½:1.
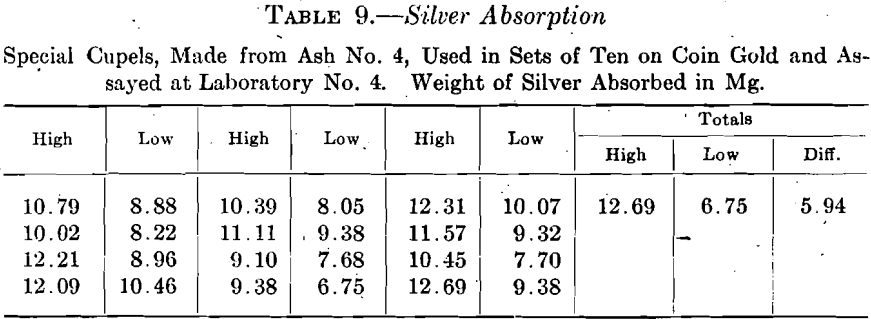
Only a single set of ten cupels made from ash No. 5, all of which passed 100-mesh was used on coin gold, 0.5 gram, silver to gold 2½:1, with the results shown in Table 10.
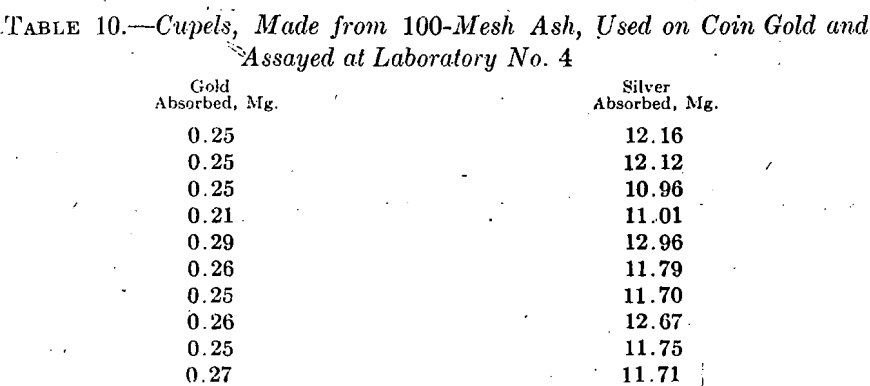
From necessity, the preliminary work of investigating cupel absorption in the Mint Bureau laboratory had to dovetail into the regular work of assaying in that laboratory. This work was then very largely on coins and included but little fine-gold assaying.
Owing to the presence of copper in coin gold and the consequent necessity of using more lead and a higher temperature in assaying, the absorptions in coin-gold assaying are not directly comparable with the absorptions in fine-gold assaying. Table 11, however, serves as a connecting link between the table of preliminary fine-gold-assay absorptions and the subsequent investigations on coin-gold absorptions in the Bureau laboratory. This table summarizes 32 coin-gold assays made in laboratory No. 5 and a set of nine coin-gold assays made in the Bureau laboratory in cupels made from a very fine ash of the following screen analysis:

All the cupels were assayed in the Bureau laboratory.
Although not strictly comparable, I insert here tests of two commercial cupels of American make, used in assaying coin gold in the Bureau laboratory:
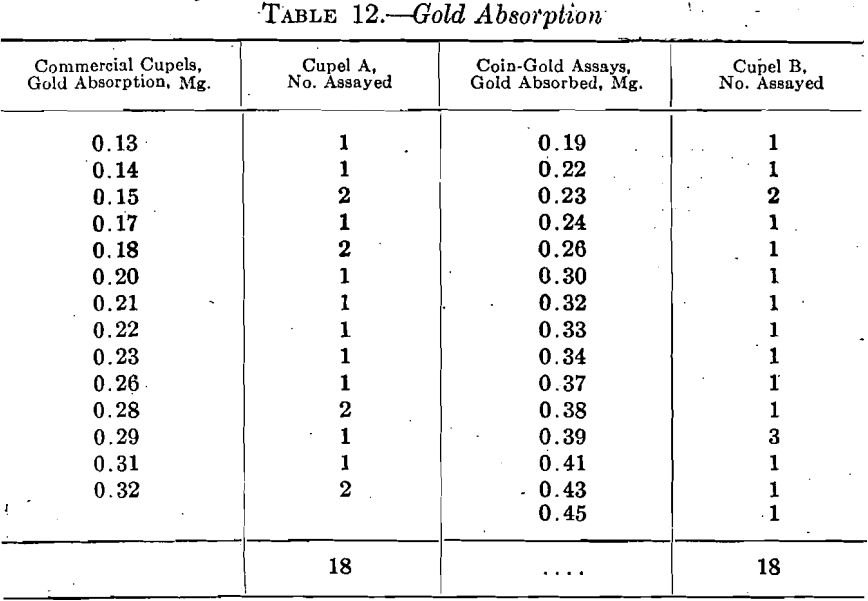
Four special samples of bone ash prepared in the Bureau laboratory and two samples of a commercial brand of bone ash yielded the figures shown in Table 13 on screen analysis.
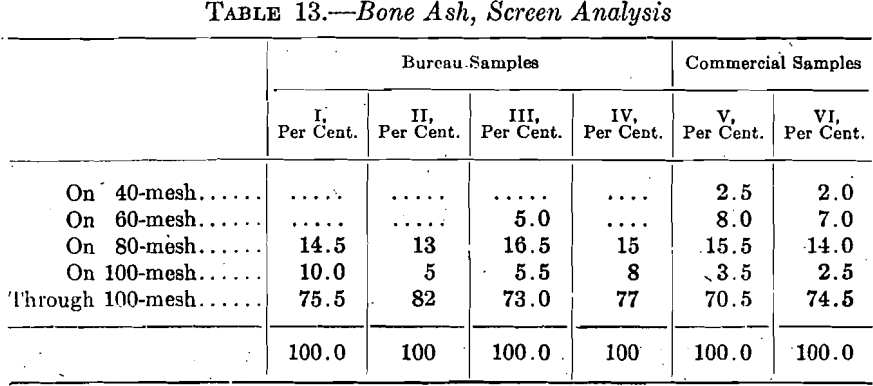
Cupels made from each of these samples of bone ash were used in the Bureau laboratory in assaying coin gold under varying conditions in groups, generally of nine. As before, the discussion of the effect of the group variations is reserved until later and all the absorptions of each type of cupel are summarized in Table 14.
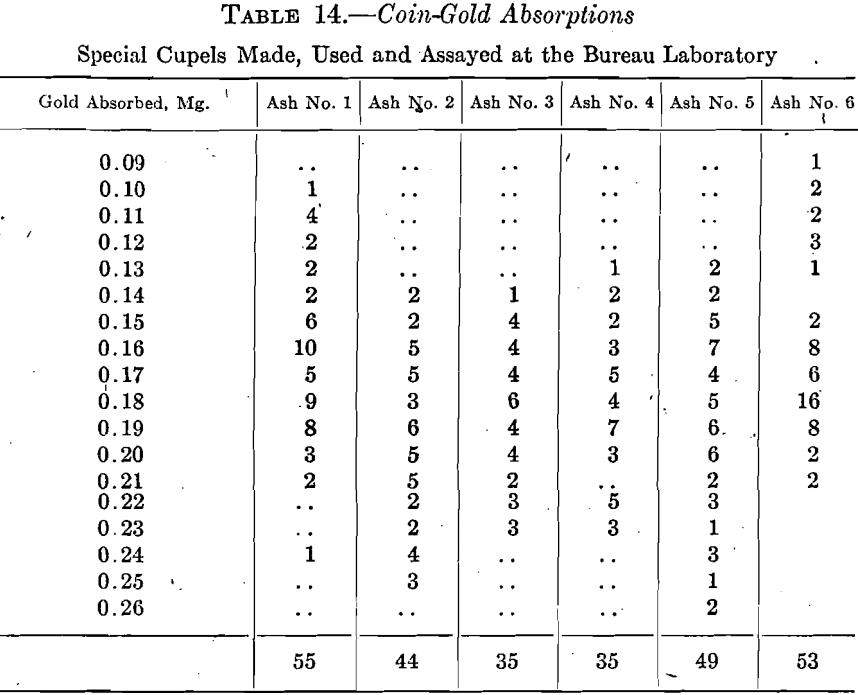
The silver absorptions for three sets of nine assays for each ash are summarized in Table 15, the proportion of gold to silver used being 1:2.25.
However, a change in the coinage laws, permitting the issue of gold certificates against gold bars as security, brought more fine-gold assaying to the Bureau laboratory, and later much work was done on cupel absorptions with this metal.
For nearly 4 years, almost all the cupelling done in the Bureau laboratory was directed toward elucidating some point regarding cupels. Besides this, many runs, both regular and special, were made in other laboratories in the service and the cupels were forwarded to the Bureau to be assayed. Also, one service laboratory made many tests and assayed the cupels themselves. In all, about 100 varieties of bone ash have been tested, and probably 10,000 or more used cupels have been assayed. The result of this investigation is a most emphatic warning against drawing conclusions regarding cupels and cupellation from insufficient data. From the vast accumulation of data now on hand, I can pick out isolated illustrations here and there to support any reasonable supposition and also a great many unreasonable and contradictory ones regarding the subject. Again I can pick out many apparent exceptions to every general rule.
The second result is to emphasize the difficulty of carrying on several sets of cupellations under even approximately the same conditions. I have tried many times to work out the effect of some chief factor in the operation to find it impossible in the end to draw rigid conclusions on account of the disturbance caused by some minor factor, which, under the circumstances, was often beyond control. It was no unusual thing for such tests to yield confusing and contradictory results. This is due to the practical difficulties of arranging and controlling the contradictory and interdependent conditions in making successive sets of cupellations.
The third result is that rigid conclusions regarding bone ash, cupels and cupellation call for such a vast amount of painstaking detailed work under such onerous conditions that the game is scarcely worth the candle, especially as the customary variations occurring in practical work will nullify many such conclusions.
Finally, then, the results of this work cannot be dealt with in any dogmatic way, but they must be applied in a general and practical way with reasonable consideration and caution.
It is, for instance, most undoubtedly true that high temperature increases cupel absorption, other things being equal, but it is seldom that all the other things are equal and it is often impossible to tell wherein they vary. It is perfectly reasonable to assume that the back part of a muffle is hotter than the front. In fact, as long ago as 1891 Roberts-Austen reported determinations by T. K. Rose showing that the back of the muffle in the British Mint was from 50° to 60° hotter than the front and that there was a difference of approximately 5° between the rows of cupels. Yet every experienced bullion assayer at times finds the loss in weight in the back cupels less than in the accompanying front ones. The same thing appears in the cupel absorption of gold and silver, and in the illustrations given below there are many instances where a cupel absorbed less than those in front of it.
It is also self-evident that the porosity of the cupel should exert an important influence on the absorption of gold. It goes without saying that cupels should possess a proper porosity, but in the vast majority of cases very little, if any, real information has been given by writers upon assaying as to just what proper porosity may be. It is generally described in some vague way as “not too fine and not too coarse.”
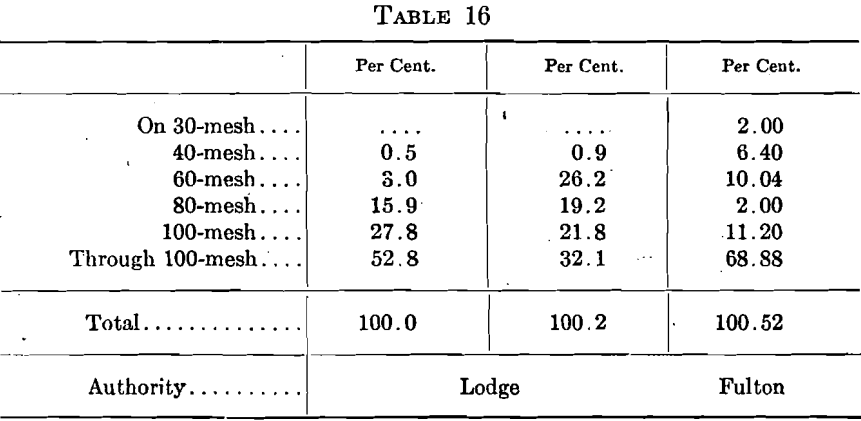
Two screen analyses of commercial bone ash are given by Lodge, which “give some idea of the bone ash on the market as passing a 40-mesh screen,” but he gives no indication of the value, relative or otherwise, of the two samples. Fulton gives “ a screen analysis of the bone ash commonly purchased, but which is rather coarse.” These three screen analyses are shown in Table 16.
It should also be noted that the pressure used in making a cupel influences the porosity.
As a general proposition, cupels must absorb the PbO as formed, but this is not governed solely by the porosity of the cupel. Size of buttons, temperature and degree of saturation of the cupel are important factors in absorption. Even yet I am not prepared to define, except in a most general way, and largely as a matter of opinion, either a maximum or minimum desirable degree of porosity, because the following tests show that its influence in bullion assaying may be so easily overbalanced by other conditions.
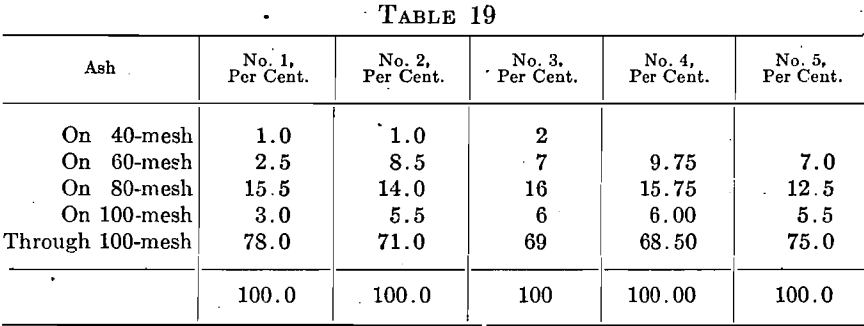
Three grades of cupels were made from five different bone ashes by the application of different pressures in making the cupels. The soft ones were made on a hand press. The standard ones were made on a power press at the usual pressure and the hard ones at a greater pressure.
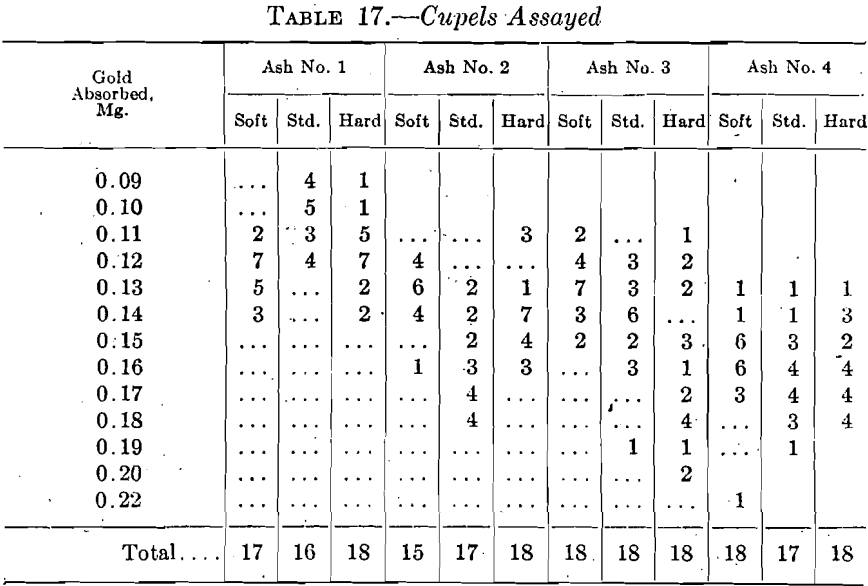
In general, 18 assays, in two sets of nine each, were made in the Bureau laboratory on each kind of cupel on coin gold, with 4 grams of lead and 2.25 parts of silver to 1 of gold; the cupellation generally occupying from 9 to 11 min. from the time the last packet went into the cupel to the time the first cupel was removed from the furnace. Several cupel assays were lost and one group is short a set of nine. The results are summarized in Table 17.
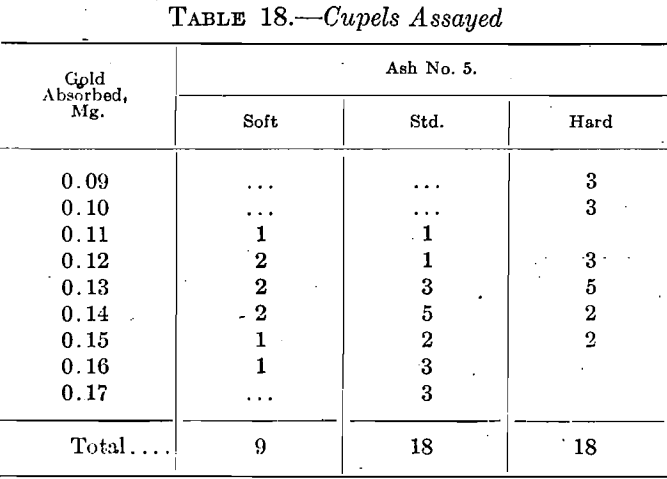
The fifth set yielded some very peculiar results. Unfortunately, only one set of nine was run on the soft cupel. In one set on the standard cupel the time was only 8 min., indicating a somewhat hotter muffle, and the same fact is reflected in the absorptions as only one is 0.14 mg, and all the rest of this set of nine are above 0.14 mg. The low results given by the hard cupel are also noticeable. Table 18 summarizes the results.
These five ashes showed the following screen compositions.
Laboratory No. 4 prepared some “very soft” and “soft” cupels from an ash of the following screen analysis:
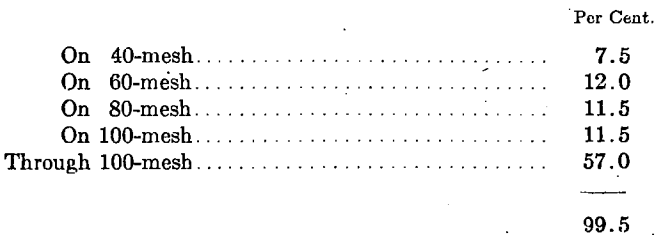
Each cupel was used in four sets of nine each in assaying fine gold with the results shown in Table 20.
The most diverse practice obtains as to the time cupels should stand and season before being used. In the British Mint it is, or was, the practice to season cupels for 2 years. On the other hand, a very experienced assayer has told me that absolutely no seasoning is required provided care is exercised in drying out new cupels in the furnace. If in a hurry he does not hesitate to take freshly pressed cupels, dry them carefully, and use them within a few hours of pressing.
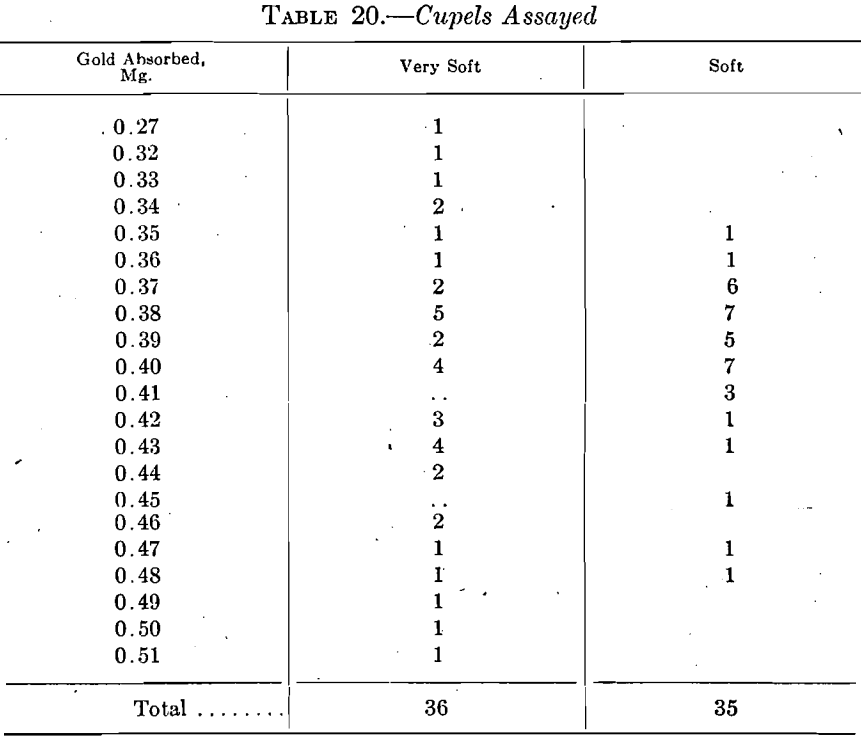
Laboratory No. 4 combined some of its porosity tests with age tests. Sets of ten cupels each, of soft and hard cupels, were used in assaying standard gold when 1, 2, 3, and 5 days old. The used cupels were assayed with the results shown in Table 21, the soft cupels 1 day old giving the lowest absorption.
These cupels were made in a power press from ash of the following screen composition:
 Laboratory No. 5 used two different cupels when 2, 8, 21, and 40 days old, in sets of nine in assaying coin gold. The used cupels were assayed at the Bureau with the following results:
Laboratory No. 5 used two different cupels when 2, 8, 21, and 40 days old, in sets of nine in assaying coin gold. The used cupels were assayed at the Bureau with the following results:
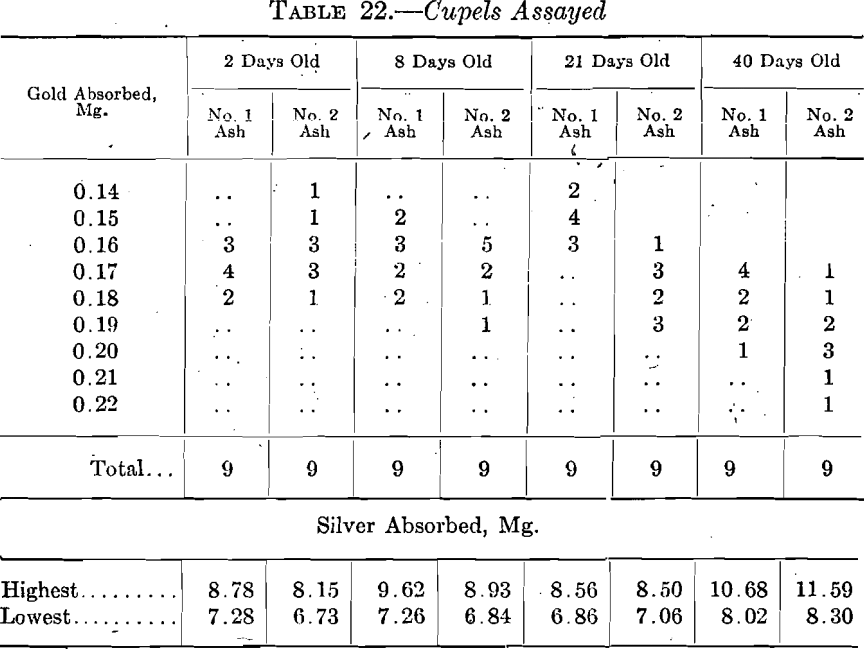
These cupels were made on a foot press from ashes of the following screen analysis:
In the Bureau, sets of nine cupels were run at 2, 8, 9, 17, and 33 days on coin gold with 4 grams of lead and 2.25 parts of silver to 1 part-of gold. The 9-day run was a temperature test rather than an age test and the muffle was about as cool as it was possible to have it safely. The absorptions are, therefore, low. A second set was run at 33 days with the furnace perceptibly hotter than on the first run, and this fact shows in the absorptions.
Table 24 summarizes the results.
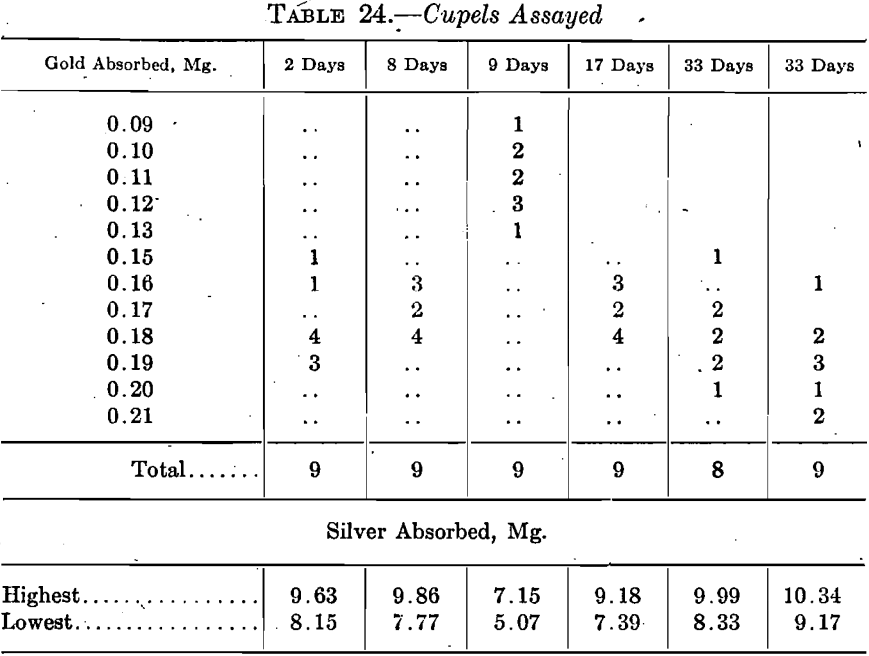
These cupels were made on a hand machine from bone ash of the following screen analysis:
It is a fair general inference from these porosity and age tests that in bullion assaying the porosity may vary greatly and that no seasoning of the cupels is required.
In considering the question of the amount of precious metals shown by assaying used cupels it must not be forgotten that distinct beads of precious metals sometimes remain on the surface of the cupels. Manifestly these must not be considered as part of the absorption. This point has been carefully watched and as far as possible beaded cupels have been eliminated from the tables or their presence noted. With the great care exercised, however, it is evident that at times the results are vitiated by minute beads. When, for instance, one cupel in a set of nine or twelve shows abnormally high results it is fair to assume that beads were present which escaped detection before assaying the cupel.
The question of the cause of beads in cupels is worthy of careful and systematic study, but it is rather difficult to work out because there is now no known means of telling when beads are likely to form in regular work, and when they are found at the end of the operation it is too late to detect the cause, in most instances, with any certainty. At this time I wish simply to mention particularly one phase of the beaded condition that affects some of the comparisons dealt with in this paper. This is that small separated beads may differ widely in composition from the main button. Thus, when the packet is melting down, a particle of bullion may get separated on the side of the cupel before becoming alloyed with any silver, while also a particle of silver may get separated before alloying with any gold. Partial alloys may also separate as beads. When the metals are thoroughly alloyed, any bead that may separate should be of the same composition as the main button.
When, for instance, one cupel in a set of nine or twelve shows an excessive amount of gold as compared with the surrounding cupels, it is a fair assumption that a bead high in gold has separated. The same reasoning would apply to silver, but the excess of silver must be very much greater to warrant the assumption of the separation of a bead rich in silver, on account of the greater amount absorbed and wider variations naturally shown. When, however, such a Cupel shows an excess of both gold and silver, it is fair to assume that a bead separated after alloying of the metals.
In a set of four rows of four cupels each used in assaying standard gold, 15 of the gold absorptions varied from 0.47 to 0.57 mg. while the silver absorptions varied from 19.50 to 23.35 mg. The sixteenth cupel, however, showed a gold absorption of 0.87 mg. while the silver absorption was 21.30 mg. Evidently a minute bead of gold separated in this cupel. In a row of three cupels used on crude bullion, the figures indicate the separation of a bead of bullion by the following absorptions in milligrams:
Gold 0.54; silver 7.02. Gold 0.84; silver 8.25. Gold 0.51; silver 6.78.
In three rows of three cupels the separation of beads of fully alloyed metal is indicated by the following absorptions in milligrams.
Owing to the very high proportion of silver in the button, from bullion assay cupels, the gold often separates in a finely divided state on parting. Even with the utmost care, a minute amount may be lost in decanting the nitrate of silver solution. Some of the discrepancies shown in the comparisons may be due to the loss of 0.01 mg. or possibly 0.02 mg. from this cause. This weight is only a very minute volume of gold. The general tendency of cupel assays is to give low results rather than high ones.
It very soon became evident in our investigations of cupels that the question of the temperature of cupelling was a most, important one. It is the most troublesome variable in assaying. It is the most difficult condition to control and to duplicate. It is probably the most potent factor in its effect on cupel absorption. At any rate, small differences of temperature may at times reverse the relative normal rate of absorption. The temperature that controls the results and success of a cupellation is the temperature of the cupelling lead button. There is no method available for determining this temperature and it may vary in adjoining cupels. It is entirely different from any pyrometer reading that can be ascertained in practical work. In a vast majority of cases, there is no relation between the two temperatures. The pyrometer reading may be maintained practically uniform while the actual temperature of the cupelling buttons may vary greatly from time to time during a cupellation. Probably the chief cause of variation, between the two temperatures lies in the amount of oxygen supplied to the cupelling bead, which is influenced both by the natural draft conditions of the muffle and the freedom with which air is allowed to enter the muffle. Again, it takes an appreciable time for changes in the heat conditions to show their effect upon the pyrometer couple while the same changes may instantly affect the temperature of the button. In working with a closed muffle door, for instance, the opening of the door will instantly cool the cupelling head by the inrush of cold air. On again closing the door the bead temperature will rise, but if the open door continue a short time only the cooling effect may not appear on the pyrometer until after the closing of the door and we will have the anomalous condition that while ( the temperature of the bead is rising the pyrometer reading is actually falling.
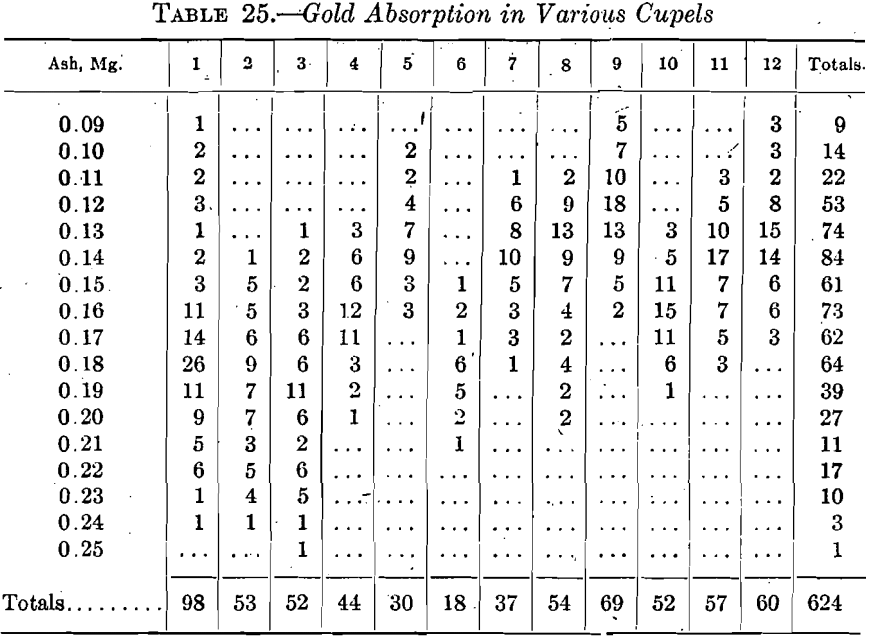
In the early stages of coin work, it looked as though certain cupels were distinctly better for this class of work than others. At that time it seemed that a small quantity of 40-mesh stuff in the ash was essential to the best work. As more data accumulated, this conclusion was weakened and was finally entirely abandoned. Table 25 summarizes the results on 624 cupels used on coin gold and made from 12 varieties of ash of widely varying screen composition, No. 1 containing 2 per cent, of 40-mesh, No. 2 all passing 40-mesh and No. 3 all passing 60-mesh.
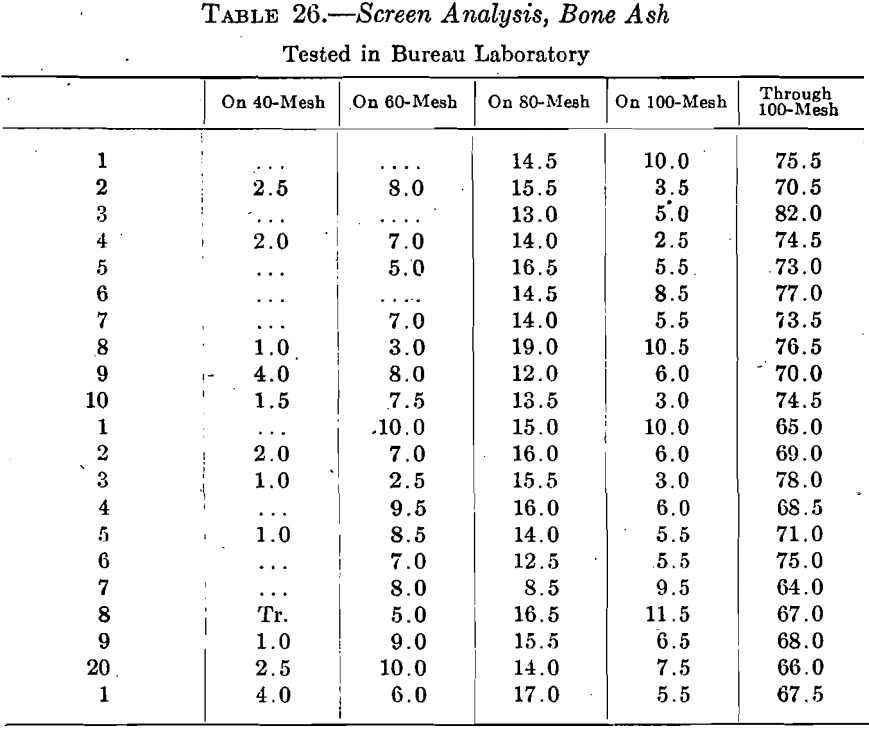
The original coin assays were made under usual conditions, but with better observation and more control of these conditions than is generally exercised. In several cases, the same cupel gave higher absorptions under different conditions, showing conclusively that the conditions of the cupellation had more influence on the absorption than the character of the cupels. This is strengthened by the fact that, on selection, sets of nine assays, three rows of three each, did not exceed an average abrsorption of 0.13 mg. per cupel on 11 varieties of ash. On raising the average to a bare trifle above 0.19 mg. the varieties of cupels are raised to 21. Table 26 gives the screen composition of the 21 ashes.
On the other hand, variations in the conditions, which were within the ordinary limits, at times brought the average up to 0.30 mg.
Many direct comparisons were made by running two or three varieties of cupels in the same set. Most of these showed practically uniform absorptions. One set of twelve, three rows of four, using three cupels gave the characteristic figures in Table 27.
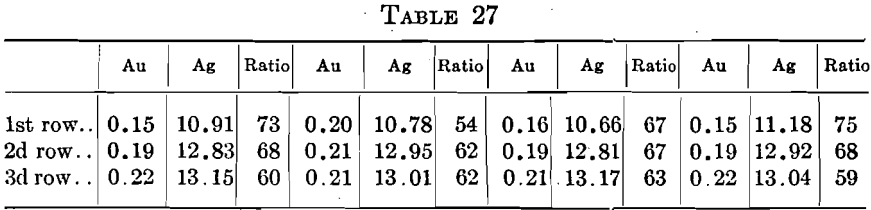
The fact that the conditions are more potent than the structure of the cupel is shown in a negative way by the silver absorptions. In sets showing low and uniform gold absorptions, the silver results vary greatly. There is an entire lack of relation between the gold and silver absorptions under normal conditions. In Table 27, the gold absorptions varied from 0.15 to 0.22 mg. while the silvers varied from 10.66 to 13.17 mg. and the parts of silver absorbed per part of gold varied from 59 to 75.
Coin gold was run in 21 sets of, twelve cupels, three rows of four each, and five kinds of cupels were employed. The general condition of the work may be taken as typical and the variations in the conditions as being within the limits of ordinary practice.
The assay of one cupel was lost entirely. Of the remaining 251 assays, 17 were thrown out for irregularities and these rejections were governed by the ratios on the surrounding cupels rather than by the absolute ratios shown. For instance, in one row the gold absorptions were 0.15, 0.20, 0.16 and 0.15 mg. The ratios of gold to silver were 1 to 73, 54, 67 and 75. Therefore, this 54 ratio was thrown out although there are five other 54 ratios in the lot. There are a few exceedingly high ratios, but I have no reason to doubt them. In one row the golds were 0.12, 0.12, 0.18 and 0.10 mg. Clearly the 0.18 is, excessive and was rejected. The remaining three ratios were 1 to 99, 91 and 108. In another case the golds were 0.13, 0.15, 0.11 and 0.12 mg. Rejecting the 0.15 mg. the ratios are 1 to 91, 100 and 95.
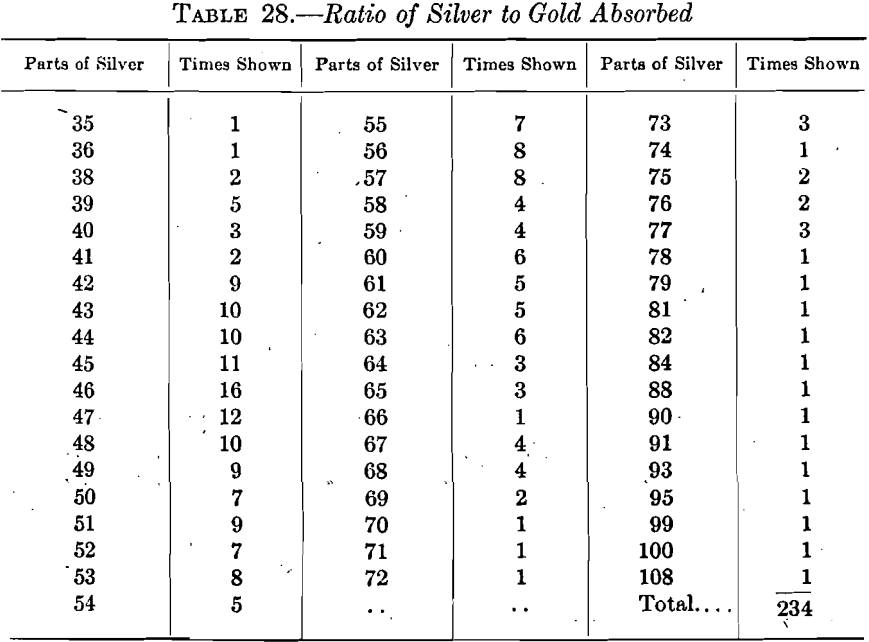
The 234 accepted assays yielded the ratios shown in Table 28, the figures stated being the parts of silver absorbed for 1 part of gold:
In the early days of the investigation, some work was done on the question of the absorption of gold by the secondary cupels. In the case of acceptable coin- and fine-gold assays, the amount of gold absorbed by a single secondary cupel is very small and it is only by uniting several that sufficient gold for satisfactory weighing can be obtained, thus averaging the results. In the second cupelling, the ratio between the gold and silver introduces a variable uncertainty from the varying protection of the gold by the silver. Again, with a wide difference in the weight of the primary cupel there will be a corresponding difference in the weight of lead cupelled in the secondary. It is therefore apparent that much work would be required to reach any definite figures upon the subject. Two cases may be given as follows:
The stained portions of nine primary cupels weighed 49.7 grams and yielded nine buttons totaling 0.87 mg. gold and 47.65 mg. silver. The stained portions of the nine secondary cupels weighed 4.66 assay tons. Two charges of 2 assay tons each were put through and the two final buttons parted together giving 0.01 mg. gold. In the second case, the primaries weighed 133.9 grams and the buttons gave 3.94 mg. gold and 141.14 mg. silver. The secondaries weighed 5.28 assay tons and the gold from two charges of 2 assay tons each weighed 0.03 mg.
Base metal assays of crude bullion often show the absorption of 1 mg. or more of gold and low absorptions of silver. In such cases the individual secondary cupel may give enough gold to be weighed. Eight cases yielded the results shown in Table 29.
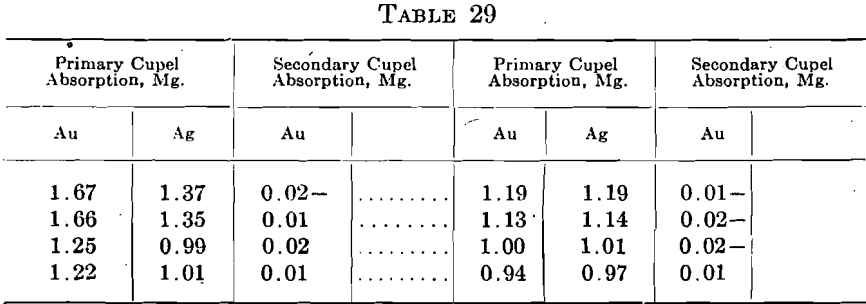
To these may be added an extreme and most unusual case of beaded cupels where both the gold assay and the base metal assay cupels were heavily beaded. The six primary gold assay cupels weighed 119.1 grams and yielded 10.28 mg. of gold, and 111.35 mg. silver. The six secondary cupels showed an average absorption of 0.01 mg. gold. The six primary base metal assay cupels weighed 110.1 grams and yielded 20.07 mg. gold and 18.23 mg. silver. The secondary cupels averaged 0.03 mg. gold absorbed.
In a very broad and general way, it appears then that approximately 1 per cent, of the gold present is absorbed by the secondary cupels. In a vast majority of cases, this is too small an amount to be of any moment.
In addition to its effect upon the cupel absorption of the precious metals, the temperature of cupellation is an important question as a general proposition and among other effects it is desirable to know the proportion between the lead absorbed by the cupel and that volatilized. Ten determinations of the lead in cupels used on coin gold in two laboratories showed that from 87.5 to 97.75 per cent, of the lead used had been absorbed by the cupel. It would appear from these figures that at least 90 per cent, of the lead used ought to be absorbed.
These illustrations show most conclusively that the conditions of the cupellation are far more important in influencing the cupel absorption than the structure of the cupel. When high or erratic losses are shown the used cupel should first be examined for beads. In the absence of beads the other conditions, especially the temperature, should be carefully considered before blaming the cupel.
It is evident that the screen analysis of the bone ash used for cupel making is not a dominant factor in cupelling. However, when the best results are desired it is necessary to have some standards to work to. At a conference of Mint Service assayers, the three following screen compositions were unanimously recommended as being satisfactory for bullion work.
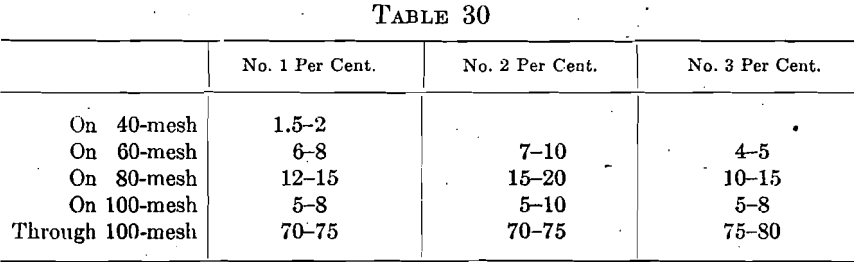
Various manufacturers were requested to bid on supplying ash graded in substantially this way and finally a bid was accepted to supply the offices throughout the service with ash graded as follows:
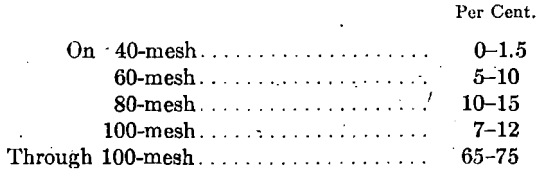
This arrangement proved unsatisfactory for reasons entirely outside the screen composition.
In the miscellaneous buying of bone ash and testing the grades supplied, it has been found that the ordinary commercial grading of ash is entirely unreliable. The Bureau tested eight lots of one grade from a manufacturer who makes a specialty of bone ash, and obtained the screen analyses shown in Table 31.
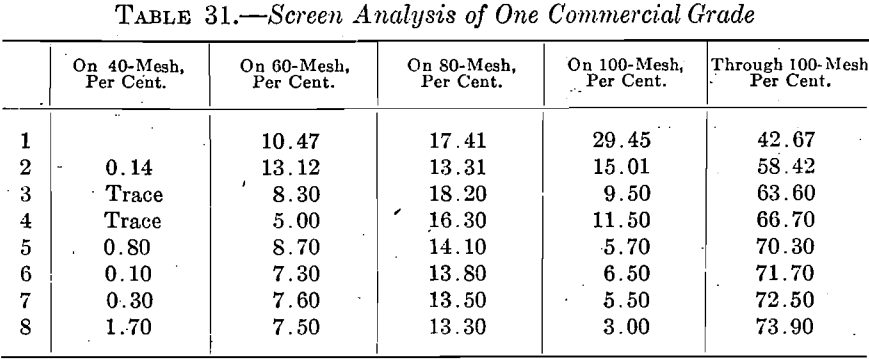
The same condition is shown by the ash of other manufacturers.
In order to overcome this difficulty, the practice has grown up of testing the ash as purchased and mixing purchases to produce a satisfactory ash. The following illustrations are taken from the practice of the New York Assay Office where this method has given much satisfaction and is to be recommended.
An ash designated as “60-80 mesh” by the manufacturer is made the basis of operations. When a barrel is received it is sampled by the use of a “tryer” and 10 oz. used for making the screen analysis. Sometimes the barrel as received compares fairly with our contract standard given above as shown by the following:
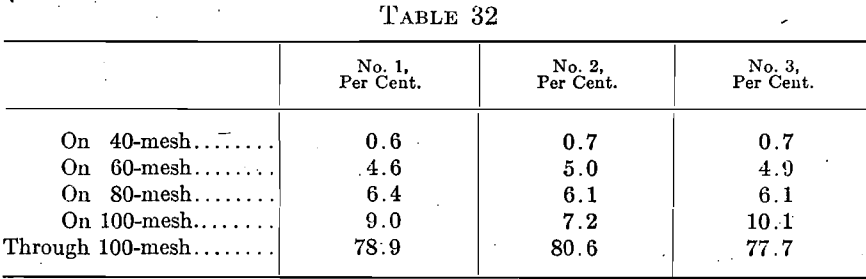
These barrels were used without mixing.
This grade is, however, frequently found to be too coarse and it is then mixed with ash graded by the manufacturer as “80-100 mesh” to give a mixture satisfactory for cupel making as shown by the following examples:
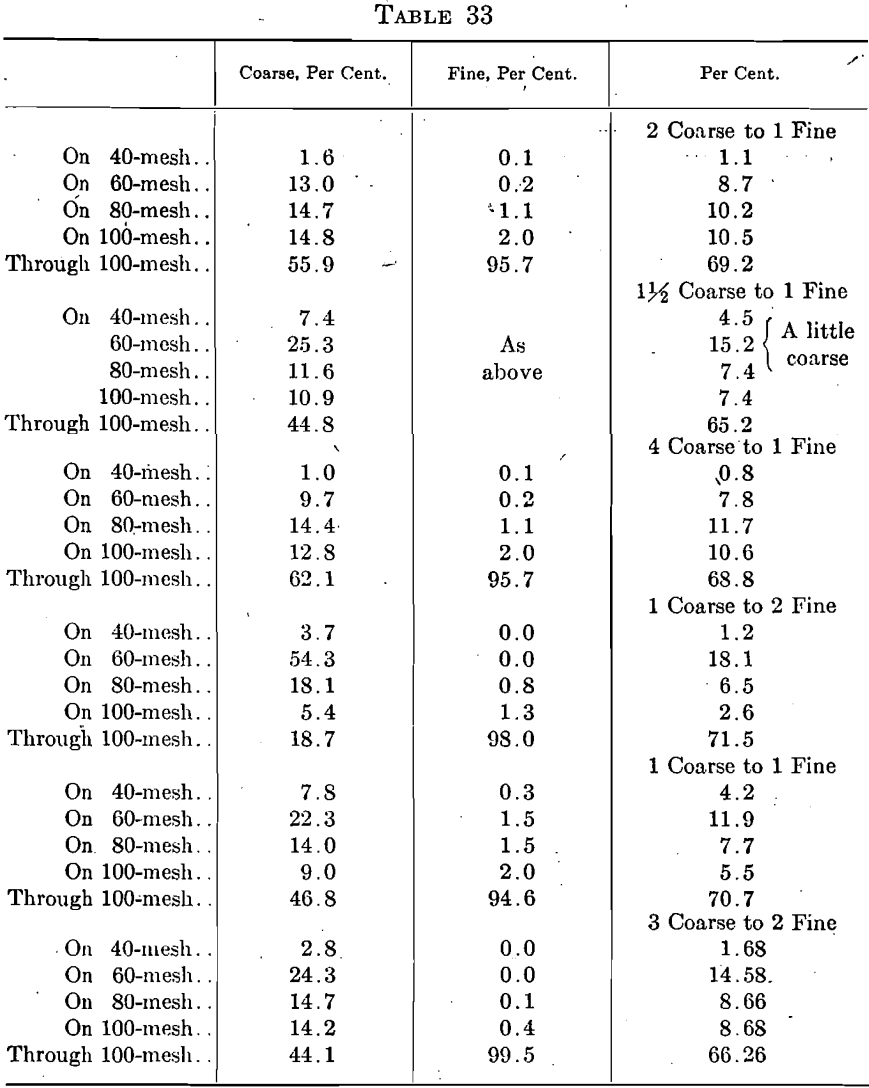
Two other barrels of “60-80 mesh” were used as received and showed the following screen compositions:
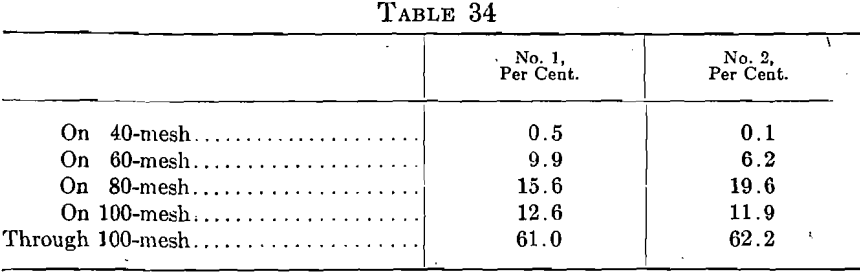
These figures also show wide variations in commercial grading.
