The processing of residues derived from various operations in the mining and metallurgical industry for by-product recovery is becoming more prevalent. A principal target for by-product recovery is flue dust produced by conventional smelting operations. Flue dust from zinc smelters has been processed for many years for the recovery of cadmium. The cadmium recovery technique which consists of a selective sulfation roast, followed by a water leach and zinc dust cementation, produces a lead cake residue, which consists primarily of lead sulfate.
The high silver content, of approximately 100 oz/ton, makes lead cake a potentially valuable product. However, bismuth, even at levels of 0.05%, frequently limits the marketability of this material as lead smelter feed. In this and other systems, the selective removal of bismuth from such residues is of considerable interest.
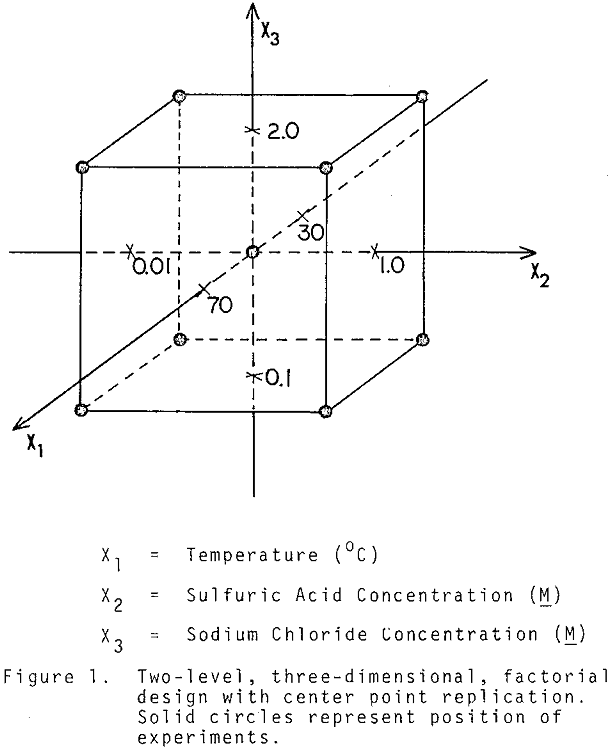
A 2³ factorial experimental design with center point replication was used to fit the model:
![]()
where X1 is the reaction temperature (°C), X2 is the acid addition (M), X3 is the sodium chloride addition (M), and a β0, β1, β7 are adjustable constants. The system response, Y, was chosen in such way that it measures the selectivity of bismuth removal with respect to silver.
Because of the large number of parameters in the empirical model, the power of the test for goodness of fit is not very high. A further attempt to verify the model by comparing the experimental response at (70, 0.5, 0.1) with the predicted response suggested that this very simple model may be inadequate for truly accurate predictions. These results suggested that the introduction of quadratic terms in equation 1 may improve its predictive capability. Additional experiments required to expand the model to include quadratic terms were not deemed justified in the present study.
Although most of the metallic constituents of lead cake form complex chioro-anions as evidenced by the stability constants, the lead complexes are generally less stable than the other complexes. This condition coupled with the low solubility of PbSO4, in sulfuric acid solutions suggests that lead solubility will be low in sulfuric acid solutions containing small additions of sodium chloride.
On the other hand, bismuth and silver both form strong complex chloro-anions suggesting that a selective separation between the two would be impossible. Such would be the case only if the chemical potential of each component in the solid phase were equivalent and if dissolution rates were the same. Although the solid solubility of bismuth and silver in the lead sulfide lattice is well known the disposition and chemical potential of these components in a lead sulfate matrix has not been determined. If the bismuth and silver assume a chemical potential similar to that which exists in their respective oxides or sulfates, then simultaneous dissolution of both components in an acidic chloride leach should occur.
Selective bismuth removal from lead cake is demonstrated by the previous results in which optimum conditions of 70°C, 1M H2SO4 and 0.1M NaCl resulted in 86.33% bismuth removal and a recovery of 99.65% silver in the residue.