Table of Contents
This paper presents results of ongoing research to develop an in-situ leaching process for Michigan copper sulfide ores. The objectives of the work presented here were to determine if the copper sulfide was extractable by a bioleaching approach and to investigate how this process could be incorporated into an in-situ leaching operation.
Laboratory Bioleaching Bacteria Selection
Eight strains of Thiobacillus ferrooxidans bacteria were obtained and subjected to growth tests. The growth rates were determined at the optimum growth temperature (28-30°C). For some strains higher (35°C) and lower (15°C) temperatures were also used.
One strain was found to grow at both 15 and 35°C, although its growth rate was about half that obtained at the optimum growth temperature of 28-30°C. Approximately the same cell densities (1 to 2 x 108) cells/cm³ were obtained with all strains at all temperatures, although the incubation periods were 2-4 times as long at 35°C and 15°C as at 28-30°C. When copper was added to solutions containing Thiobacillus ferrooxidans bacteria, only one strain grew well at the highest copper level (20.2 g/L). However, others were able to adapt to slightly lower copper concentrations (15.2 g/L). Studies conducted on the most copper- resistant culture, which had been adapted to 20.2 g of Cu/L (CuA2) to determine if it could use Cu+¹ as an energy source, produced no evidence of Cu+¹ oxidation at either 1 or 5 g/L CuCl levels in solution.
Bacterial Assisted Bioleaching Experiments
The leaching set-up consisted of pairs of glass columns, one containing ore and the other containing bacteria culture on an inert media. Laboratory pumps moved the lixiviant (acid ferric sulfate solution) from a 2.5 L reservoir to the ore column where the liquid level was held above the crushed ore to simulate saturated in-situ conditions. During the tests the lixiviant passed through the ore column and returned by gravity flow to the reservoir. As copper was solubilized, iron was converted from Fe+³ to Fe+². Bacterial regeneration of Fe+³ was accomplished by pumping liquid from the common reservoir into the SBC column from where it returned to the reservoir also by gravity flow. A schematic of the leaching set-up is shown in Figure 3.
Copper Bioleaching Test Conditions
Eight pairs of columns were used to test a variety of leaching conditions. The test matrix is listed in Table 1. Six pairs were used to test the Keweenaw ore under various conditions and the other two pairs were used to test two other Michigan ore types (“White Pine” and “Kona”) under “standard conditions” of bioleaching (Test 1 of Table 1 ).
Ore samples were crushed and 600 g samples of -4+10 mesh material were initially leached to extract acid-soluble copper (oxides, carbonates, etc.).
Six of these were samples of the Keweenaw ore (identified as Bohemia) to investigate the effects of test conditions on the leach rate and the ferric sulfate regeneration rate.
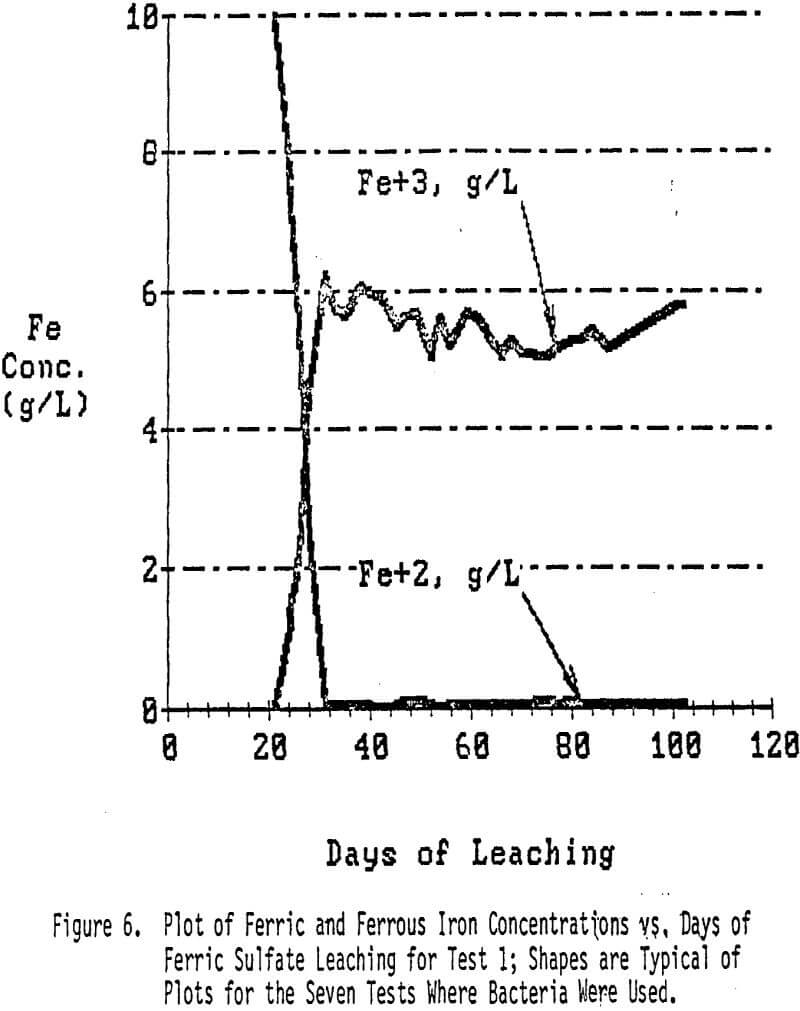
The leach solutions were analyzed at regular intervals to determine the rate of copper dissolution and the point in time at which little or no more copper dissolution would take place. When the rate of copper dissolution by acid alone had slowed to nearly a halt, the pregnant solutions were removed. Fresh leach solutions containing ferrous sulfate and Thiobacillus ferrooxidans bacteria were then added to continue leaching the remaining copper, most of which was in the form of the insoluble-in-acid Cu2S (chalcocite).
This change to bacterial leaching required the addition of the SBC column for each leach test. The leach solutions and air contact the bacteria growing on the surfaces of the SBC media either by alternate filling and syphoning of the SBC column or by trickling the solution over the media and draining it out the bottom of the column into the reservoir.
Leaching under saturated conditions with bacterially-assisted ferric sulfate regeneration was continued throughout the test. The leach solutions were reacidified to a 1.8 pH level with 0.1N H2SO4 as needed. Samples were taken and analyzed for copper and for ferrous and ferric iron approximately every 2 to 3 days.
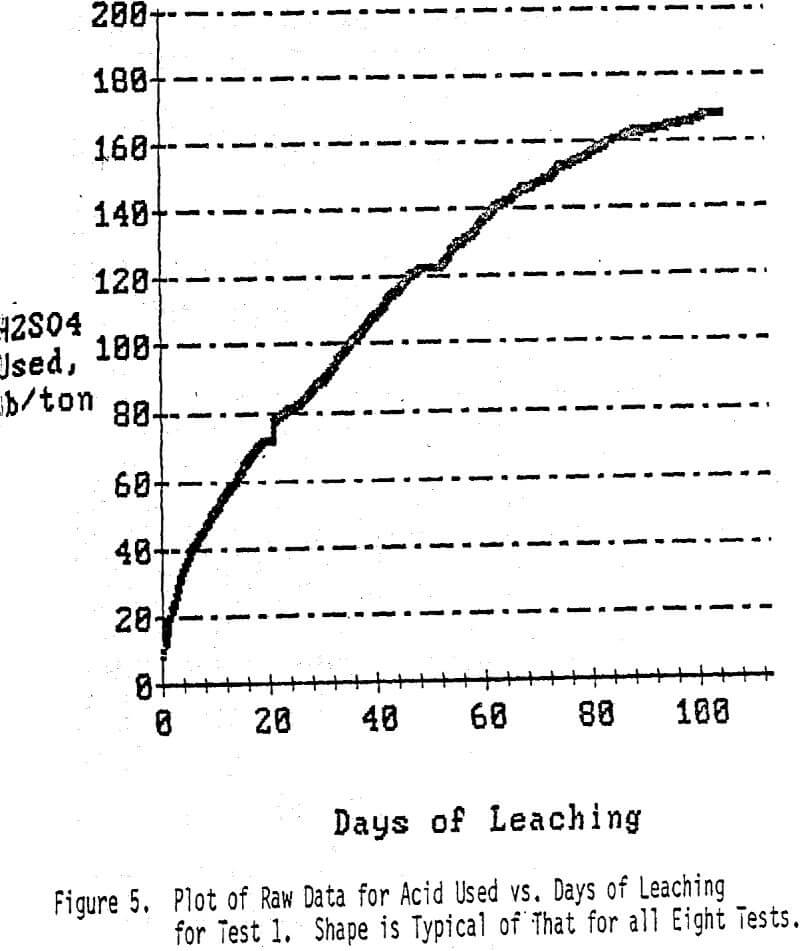
The acid leaching phase took about 19 days. At this time iron sulfate and bacteria were added and leaching was continued for an additional 84 days. At this time it was established that the copper dissolution was essentially complete. Actually, there was little dissolution of copper during the last ten days of the test.
