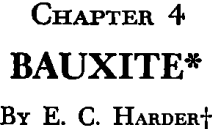Table of Contents
Bauxite is known mainly as the ore from which aluminum is smelted but it has large use also in the manufacture of artificial abrasives and as a basis for certain chemical industries. A small amount is used for refractories and for other purposes.
Composition
Dana and others give the mineral formula of bauxite as Al2O3·2H2O and the composition as Al2O3, 73.9 pct; H2O, 26.1 pct. It has been definitely shown, however, that bauxite does not exist as a specific mineral but rather as a rock. The term is now used synonymously with aluminum ore and it embraces gibbsite (hydrargillite or alpha trihydrate), Al2O3·3H2O (Al2O3, 65.4 pct; H2O, 34.6 pct); boehmite (alpha monohydrate), Al2O3·H20(Al2O3, 85 pct; H2O, 15 pct); diaspore (beta monohydrate), Al2O3.H2O(Al2O3, 85 pct; H2O, 15 pct), and mixtures in various proportions of any two of them. Bauxite of the Mesozoic and Cenozoic in Europe is predominantly a mixture of gibbsite and boehmite, subordinately a mixture of boehmite and diaspore, or gibbsite and diaspore. The bauxite of North and South America, tropical Africa, Asia, and Australasia consists largely of gibbsite. Corundum (Al2O3) is not included under bauxite, although gradations from bauxite to emery or corundum exist.
Properties
Gibbsite is white or light shades of gray, cream or pink. Its hardness is 2.5 to 3.5; specific gravity, 2.3 to 2.4; crystal system, monoclinic, crystals tabular parallel to 001, often fibrous, concretionary, stalactitic; luster, vitreous to pearly; generally translucent; cleavage, parallel to 001; disassociation temperature, approximately 300°C.
Boehmite, a new mineral described by J. Boehm in 1925, confirmed and named by J. de Lapparent in 1930, has some properties not yet definitely determined. It occurs in grayish, brownish and reddish shades. Its hardness is between that of gibbsite and diaspore; specific gravity, 3.01 to 3.06 (artif.); crystal system, orthorhombic, crystals lenticular tabular, best developed along 110 and 001; angle between the faces m near 63°; cleavage, parallel to 010; X-ray pattern distinctly different from that of diaspore.
Mode of Occurrence
Bauxite deposits occur in a number of different geologic associations but most of them can be grouped into four main classes:
- Blanket deposits occurring at or near the surface in horizontal, tilted, or undulating sheets and lenses.
- Beds and lenslike deposits occurring at definite stratigraphic horizons interlayered with sediments or between sediments and igneous rocks.
- Pocket deposits or irregular masses enclosed within limestone or clay or igneous rock.
- Detrital deposits formed by the mechanical breaking up, transportation and redeposition of the material from deposits of the other three types.
These four types of deposits are closely related to each other and are in many cases gradational. There is a strong possibility that the first three have a somewhat similar origin. Table 1 shows the general age relations of the principal bauxite deposits of the world.

Origin
The hypotheses that have been advanced to explain the origin of bauxite may be grouped under four main headings: (1) chemical sedimentation, (2) chemical replacement, (3) weathering in situ, (4) detrital deposition.
Many geologists have considered it necessary to explain the formation of bauxite deposits by processes of solution and redeposition. Igneous rocks and clays are looked upon as the source of the aluminum. As solvents, various acids, alkalies and salts were taken into consideration —for instance, nitric acid, derived from bacterial activity, or from lightning discharges; sulphuric acid resulting from the decomposition of sulphides; sodium chloride from marine waters, and sodium hydroxide or sodium carbonate obtained by decomposition of various rocks. Hot springs are called upon as agents. Deposition is believed to occur in enclosed basins by reaction with carbon dioxide, organic acids, hydrogen sulphide or calcareous waters, with accompanying cooling.
Distribution of Deposits
Although bauxite deposits occur abundantly in many parts of the world, the mining of bauxite in important quantities is confined to relatively few countries. Roughly, in the order of their importance during recent years, the principal bauxite-producing countries are United States, British Guiana, Surinam, France, Hungary, Russia, Netherlands Indies, Yugoslavia, Gold Coast, and Italy. Minor quantities of bauxite have been produced in Brazil, Greece, India, Malaya, Northern Ireland, Germany, Caroline Islands, Rumania, Spain, and Australia.
Production and Consumption
Although bauxite was discovered in France in 1821, there is no record of any production until 1873, when 200 tons was mined. At the end of the century the output had not reached 50,000 tons. Thereafter, however, bauxite mining progressed rapidly and in 1913 more than 300,000 tons was produced. In the United States, the first bauxite production dates from 1889, when 728 tons was mined in the Rome district, Georgia: The Arkansas bauxite mines began producing in 1899, with an output of 5045 tons in that year. Until World War I started, more than 95 pct of the bauxite produced annually came from the mines of France and the United States. During the war, however, the necessity for the opening up of new bauxite deposits became apparent and while the deposits of Istria and Dalmatia were being developed by the Central Powers the Allies turned their attention to the newly discovered deposits of British Guiana and Surinam. The British Guiana mines started shipments in 1917 and the Surinam mines in 1922. Somewhat later, bauxite was discovered in Hungary and was developed for the benefit of the German aluminum plants. Bauxite production began in Russia in the late twenties. During the past 15 years it has continued at a high and increasing rate.
Fig 1 shows graphically the bauxite production from 1935 to 1946 in the principal producing countries.
Prospecting and Exploration
Although bauxite deposits were formed under conditions existing in warm climates, they are not necessarily confined to tropical or subtropical regions, owing to variations in climatic conditions in past geologic times. Thus the Arkansas bauxite deposits occur at latitude 35 °N, those of southern Europe between 40° and 50°N and those of the Tikhvin region in Russia at latitude 60°N. Bauxite deposits usually are discovered from outcrops and fragments of float. Rock associations, however, may serve as a guide, bauxite being commonly found with clay, limestone or certain igneous rocks such as syenites, feldspathic schists, dolerites and basalts. Probably the most striking association of bauxite is with old land surfaces. Thus bauxite deposits in the older rocks occur along unconformities representing long time intervals, while those of recent origin occur on moderately level, peneplained surfaces that have been undisturbed for a long period of time.
Once found by outcrops, bauxite deposits are explored by drilling. It is generally preferable to use hand drills, as most bauxite deposits occur in remote regions where transporation is difficult; moreover, many deposits are found at relatively shallow depths. In places, however, as on the flanks of the ore bodies or elsewhere where they are deeply buried, power drills have been used successfully, although coring generally has been found unsatisfactory. It is best to perform the exploration by drills in two stages, the early drilling, widely spaced, and merely for the purpose of determining the quality and estimating approximate tonnages; the later drilling at close intervals to serve as a guide to mining. A few test pits should be sunk on all deposits to supplement the drilling, mainly with the object of securing samples for washing and other beneficiation tests.
Mining Methods
The methods used in mining bauxite differ somewhat in different countries and with different types of deposits. In deposits occurring in the form of extensive surface blankets or in beds and lenses interlayered with unconsolidated sediments, such as are being mined in Arkansas and in northern South America, quarrying is a common method of mining. The overburden, where present, is removed by hand or by scrapers or, where thick, by power shovels or draglines, and the upper surface of the bauxite layer is thoroughly cleaned by means of picks and shovels. Holes for blasting are then drilled into the ore bed vertically, starting at the surface and penetrating into the lower portion of the bauxite layer. Generally such holes are drilled in series parallel to the existing quarry faces. After blasting, the broken bauxite is picked up by power shovels, loaded on mine cars, and transported by rail to beneficiation plants.
Where the overburden is too deep to be removed by stripping or where lenses and layers of bauxite are interlayerd with solid rock, as limestone is in the European fields, and the attitude of the enclosing rock is such that the deposits reach considerable depths, quarrying methods are used along the outcrops and underground mining methods at depths. The underground mining is advanced by a series of drifts and crosscuts and caving is employed in retreating. The bauxite is removed from the workings in mine cars, through tunnels, shafts or inclined planes, according to the form of the deposit and the method of working, and is transported from the mines to railway stations or seaports by motor lorries. European bauxite is not subjected to beneficiation.
Preparation for Market
In Arkansas, British Guiana, Surinam and the Netherlands Indies, the bauxite is beneficiated before marketing. In Europe, the bauxite is shipped direct to consuming plants in the crude state as mined. In the United States and in the Guianas the crude bauxite, as mined, consists of fines and fragments ranging in size up to a foot or more and contains 12 to 15 pct free moisture. This crude bauxite is crushed to a maximum size of 2½ to 3 in. and is then washed to remove the clay and other impurities present. Generally the washing is performed in scrubbers or trommel-rake washers but locally vibrating screens are used to supplement these.
As the washed bauxite contains about 15 to 18 pct moisture, it is dried before shipment, in rotary kilns that remove all except 1 to 3 pct of the free moisture. The rotary kilns are from 60 to 200 ft long and 6 to 8 ft in diameter, depending upon the size of the operation. They are lined with firebrick. The temperature of the bauxite leaving these kilns is about 200°F. From 20 to 50 tons per kiln hour may be produced. This is the ordinary crushed, washed, and dried bauxite, consisting of mixed lumps and fines, that is marketed for the manufacture of aluminum and for chemical, abrasive and refractory purposes. The main reason for drying the bauxite at the mines is to save transportation costs but drying is important also for the fabricating plants where the bauxite is to be consumed, as the fine grinding necessary for further treatment during processing requires a dry material.