Flocculation is the process of agglomerating fine particles in solid-liquid suspension by using a high molecular weight long chain polymer as a flocculant. It is used industrially as a pretreatment step to solid-liquid separation and also as a separation method for recovering valuable minerals from gangue using selective flocculation.
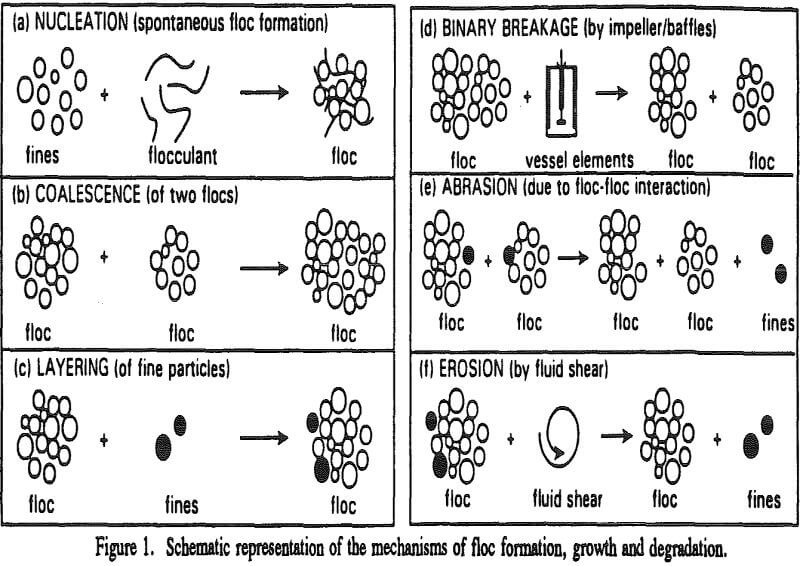
Spontaneous floc formation takes place due to nucleation mechanism. That is, on addition, dispersion and adsorption of the flocculant, many of those infinitesimally small particles “freeze” together instantaneously to form an agglomerate of some finite dimension and this is termed as nucleation.
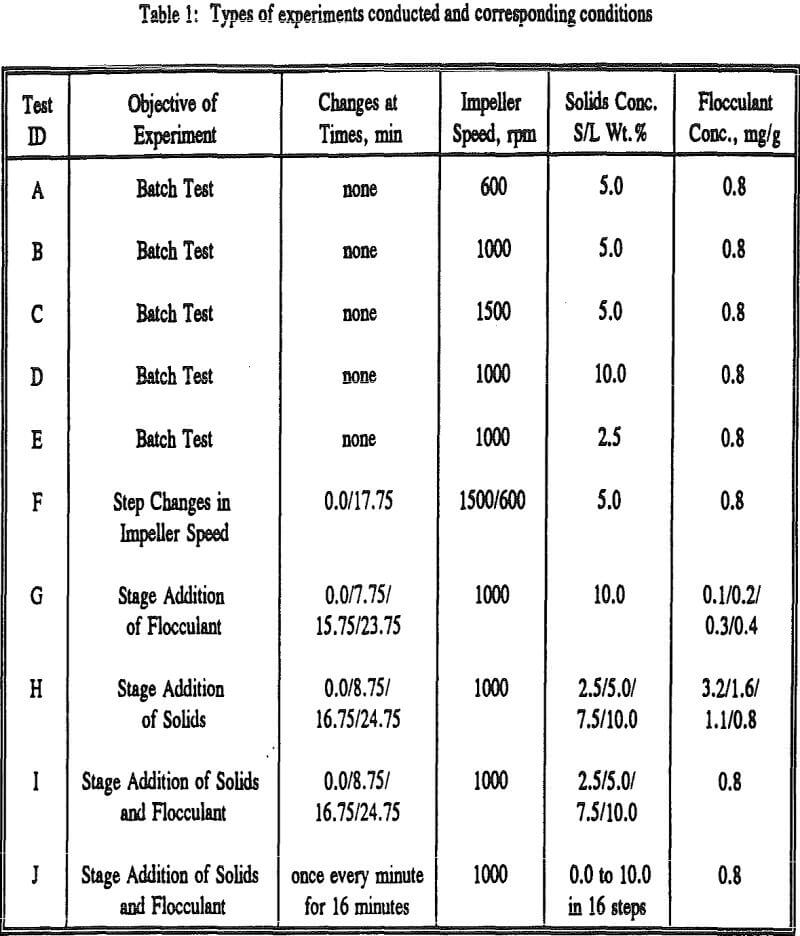
The experimental procedure involved making up the feed slurry to consist of 4 liters of distilled water and with desired concentration of solids and adjusting the pH to 10.4. The feed solids are kept in suspension by mixing at the chosen impeller speed and at time = 0, the flocculant is added. Samples of flocculated slurry were withdrawn from the tank at desired times and analyzed in the sedimentation column for the floe size distribution.
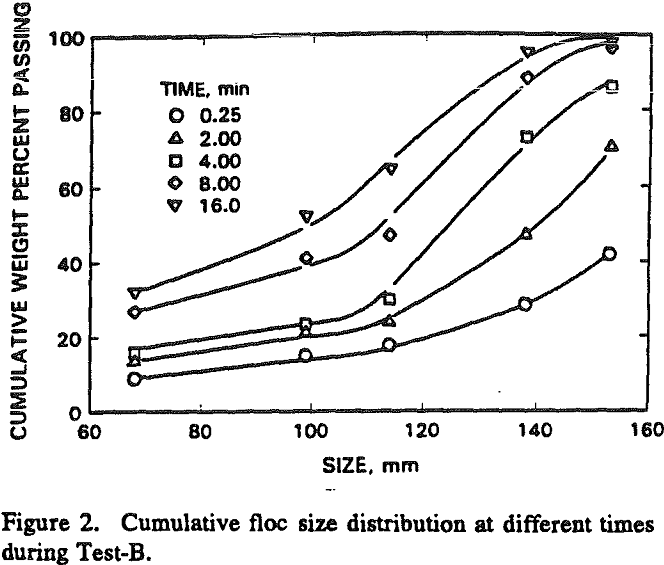
As it is difficult to analyze the effect of different variables on the basis of complete size distribution, we have chosen to use the weight fraction of flocs coarser than the equivalent diameter of 68 µm as the parameter for assessing the response of the flocculation process.
As expected, the degradation is faster at higher speeds of agitation. In all the three cases, an equilibrium value for weight percent +68 µm is reached. A step decrease in the agitation intensity is expected to bring in a definite amount of reflocculation.
When the polymer dosage is too low it may not be sufficient to form bridges between all the particles and whatever bridges are formed may not be very strong. This explains a rapid degradation of the flocs in the beginning. When some more polymer is added it adsorbs onto the free particles and to some free sites on the existing flocs thus flocculating them. Once the particles and flocs are saturated with necessary polymer, any further addition of the polymer may not cause any growth of the floes or particles, because they are already covered completely by the polymer chains.
As mentioned earlier floc formation takes place by nucleation and subsequent size changes by simultaneous occurrence of growth and degradation mechanisms. The growth of the flocs after the flocs are formed in the first few seconds is not reflected in the size distribution curves of batch flocculation because the rate of degradation is much more than the rate of growth initially.
The physical and surface chemical principles of flocculation are normally studied to a greater extent than the process engineering aspects. Most of the process engineering studies are concerned with batch systems and continuous flocculation is rarely addressed.