Table of Contents
In dealing with wolfram ores and concentrates, we identified 3 three determination methods for the estimation and Assaying Tungsten that are discussed below are well known, but these results of their trials and comparison under technical conditions may be interesting.
Tungsten Assaying Method 1
The routine procedure for the assay of rich wolfram ores or concentrates was as follows:—Take 1 gram of finely-ground sample; digest with HCl and HNO3 for about 2 hours, evaporating down to near dryness. To the remaining 5 cc., say, of liquid, add water, warm or boil for ¼ to ½ hour, filter through a small filter paper, and wash the beaker and the insoluble matter with hot dilute HCl. It is best to keep the bulk of the insoluble matter (WO3, SiO2, etc.) in the beaker at this stage. Then place a weighed porcelain dish under the filter funnel and wash the beaker and the filter paper with successive small quantities of strong NH4OH, finally bringing all the insoluble matter on to the filter paper, and collecting the ammoniacal filtrates in the porcelain dish. Then place the filter paper with the insoluble matter in a porcelain crucible, dry and burn off the paper, cool, and brush the contents of the crucible on to an agate mortar, grind it to flour, and brush this back to the original beaker without loss. In the absence of draughts this can be done safely. Re-treat this residue with acid and ammonia as above, running the second ammoniacal filtrate into the previous porcelain dish, which in the meantime has been heating on a water-bath. Finally evaporate to dryness on the water-bath the combined ammoniacal filtrate, and then heat gradually to a red heat over a Bunsen burner, cool, and weigh. Then the contents of the porcelain dish give the WO3 (tungstic acid) directly.
The final filter paper and insoluble matter may be ignited in a porcelain crucible, thus giving the “insoluble matter.” If the ore is only wolfram with insoluble matter, which is often the case, then (% WO3 x 4/3 + % insol.) should reach 100 %, and this is sometimes a useful check. Pure wolfram (FeMn) WO4, contains about 76% WO3. Some authorities quote a maximum value of 76.6.% WO3, but the Tasmanian wolfram best known to the writer contained at the most 75.8 % WO3.
Notes on Tungsten Assay Method 1
- The attack of HCl or aqua regia on wolfram produces the insoluble WO3, and thus the action is slow, and the last portion of the wolfram is liable to be protected from decomposition. Fine grinding is essential, therefore, and especially for material containing over 38 per cent, of WO3—i.e., over 50 per cent, of wolfram. The actual assay sample of such material must be ground to flour in an agate mortar. This is easier said than done. Also, during the acid treatment the sample is liable to “ crust up ” and thus to protect the lower layers from the action ; this crust should be broken up several times during the action. When products containing less than 50 % wolfram are ground, and the wolfram is the softest constituent, then a ground product is easily obtained in which all the wolfram is fine enough to be completely and readily decomposed by acids; and if the other constituents of the ore are insoluble in the acids, their presence keeps the wolfram particles separated, and considerably helps the complete decomposition of the wolfram, and in this case the second acid and ammonia treatment is unnecessary. Such common constituents of wolfram ores are silica, silicates, and cassiterite.
- The writer could see little if any advantage accruing from prolonged action of HCl alone ; therefore, after treatment with HCl for about ¼ hour the HNO3 was added and the solution slowly evaporated down. The usual amounts of acids were about 40 cc. HCl and 5 cc. HNO3, and the time of treatment would vary from 1 to 3 hours. It is doubtful if prolonged action of acid is worth the time —i.e., probably the bulk of the wolfram is decomposed within an hour. The second acid treatment, necessitated by the imperfect grinding or the incomplete decomposition in the first case, is completed in a short time—say, ½- hour. Evaporation of the acid right to dryness is liable to render the WO3 difficultly soluble in ammonia.
- It is necessary to wash the WO3 on the filter paper with strong ammonia, otherwise, colloidal silica will run through and ultimately be weighed as WO3. It is not necessary to add ammonium chloride to the ammonia wash liquor, and the presence of excessive ammonium chloride with the ammonium tungstate in the porcelain dish causes the contents to flake and jump off the dish during the ignition, thus producing mechanical loss of WO3. The ammonia washing of the WO3 sometimes becomes slow; possibly the silica (or silicic acid) clogs the pores of the filter paper. It is thus best to bring the bulk of the insoluble matter on to the filter paper only with the last ammonia washes. The ammonia filtrate should fit comfortably in an 80-cc. porcelain dish.
- The use of a water bath is recommended, as the ammoniacal liquor then evaporates to dryness quietly and safely, and needs no watching.
- In this method there is a tendency for colloidal silica to run through with the ammonia liquor, but this may be kept at a minimum by the conditions described under note (3). If the ammoniacal filtrates are collected in a platinum dish the SiO2 may be removed by the usual H2SO4 and HF treatment, and this treatment might be necessary for low-grade material containing easily decomposable silicates ; it was not adopted in the work covered by these notes.
The filtrate after the first acid treatment can be tested, after dilution, with H2S, and thus CuS, Bi2S3, etc., can be precipitated and qualitatively and quantitatively estimated, and afterwards, of course, other constituents of the ore—e.g., the calcium from scheelite, CaWO4—can be detected and estimated in the usual way.
The final insoluble matter may be inspected for silica or rock minerals (white), titaniferous iron ore (black), and especially for cassiterite (after the acid treatment, brownish). Such an inspection, after a little experience, will enable the tin content of the wolfram concentrates to be estimated to the nearest per cent., especially when the amount is small—say less than 5 % Sn. In any case the tin content of the sample may be obtained with accuracy by Pearce’s method applied to this insoluble matter—i.e., by fusing it with Na2O2, dissolving the melt in HCl, reducing the strongly-acid hot solution with nickel, cooling in CO2, and diluting and titrating the SnCl2 solution with an iodine solution which has been standardized similarly against pure metallic tin. The writer found this to be the most satisfactory method of estimating the tin in all cassiterite- wolfram concentrates.
When dealing with or purchasing various and unfamiliar wolfram concentrates, the above acid method thus enables the other constituents to be detected and estimated, if necessary, with the minimum of trouble, and this is an important feature. Nevertheless, as will be seen later, with concentrates rich in wolfram care is necessary to avoid low results for the tungstic acid content, and in general the higher of two duplicates will give the more correct per cent. WO3, The common tendency is for some of the wolfram to remain unattacked or for some of the WO3 to remain occluded as ammonium tungstate in the filter paper. In the latter case the yellow WO3 will be seen in the final ignited insoluble matter. By this method duplicate results should agree to within 1 % of the wolfram content of the sample.
Tungsten Assaying Method 2
A modification of the above method, which obviates the last-mentioned source of error, is to grind ‘perfectly at the start ‘ so as to ensure complete decomposition of the ore in one acid treatment, and, after washing the WO3 free from acid, to place it, filter paper and all, in AmOH solution in a graduated flask or beaker (the original beaker may serve), and after its dissolution as ammonium tungstate to make up to the definite volume, and then to take of the clear supernatant liquid a definite aliquot part, which, on evaporation to dryness and ignition, gives the WO3, and hence the % WO3 of the sample is calculated. This method does not necessarily demand complete disintegration of the ore in one acid treatment, but such would be advisable. Excepting that the remaining insoluble matter is not directly collected, this modified acid method is perhaps better than the previous one—i.e., it is inclined to give higher results for the % WO3.
Tungsten Assay Method 3
The writer also used the well-known mercurous nitrate method of estimating tungsten, and by this means correct results may be obtained. This method was carried out as follows :—Take ½ gram of wolfram concentrates and fuse with NaOH in a nickel dish for 15 minutes ; then dissolve the melt in hot water, a little Na2O2 being added, and then filter hot from the insoluble Fe and Mn oxides, unattacked SnO2 etc., and wash well with hot water. Then slightly acidify the filtrate with HNO3, heat till the CO2 escapes and the separated silicic acid (and stannic acid) is coagulated, and then make alkaline with ammonia, heat and filter, and wash. Again slightly acidify the filtrate with HNO3, and precipitate the tungsten as mercurous tungstate, Hg2WO4, a pale yellow coagulable precipitate,, by the addition of Hg2(NO3)2 solution. This last step demands a nearly neutral solution, but as the Hg2(NO3)2 solution can only be made with excess of HNO3, the assay solution becomes more acid, during the precipitation; small excess of Hg2(NO3)2 is added and then dilute AmOH drop by drop till the liquor is neutral or slightly acid, when all the tungsten is precipitated as Hg2WO4, along with more or less black Hg and mercury amino-compounds. As. Hg2WO4 is soluble (with decomposition) in excess of AmOH or of HNO3, the conditions of precipitation must be carefully observed, otherwise it is possible to leave tungsten in the solution. The tungsten hearing precipitate is well washed with hot water containing a little- Hg2(NO3)2, the precipitate is dried, placed in a porcelain crucible,, and ignited, and the resulting WO3 is weighed.
This is probably, when combined with suitable modifications (vide-note “ 5 ” above), the most accurate method of separation of the tungsten from all other constituents of most wolfram ores, e.g.— SiO2, SnO2, Nb, and Ta; but it is not suitable for scheelite or for ores containing Ca and similar metals, appreciable metallic sulphides, especially arsenical pyrites, or phosphates, in which cases the acid method is preferable. In the Hg2(NO3)2 method the “ fiddling about ” necessary to ensure the complete precipitation of the wolfram takes time, and also the other constituents of the original ore are not separated in a form in which they can be readily detected or estimated, and finally this method is rather expensive. These facts, and the almost general adoption of the acid method by others (buyers, or sellers), led to the restricted use of the Hg2(NO3)2 method.
The following table will indicate some results on wolfram ores or concentrates obtained by means of these three methods. The figures quoted are in all cases the means of duplicates. The results by methods (1) and (3) were obtained by the writer on the same bulk assay sample; the results by method (2) were obtained by an independent assayer on his own sample, and thus between (1) or (3) and (2) there is a possible divergence due to the two samplings, but as these were carried out on approximately 5-ton parcels of the fine material (minus 6 mesh), shipped in bags of 1 cwt. capacity, the possible errors of sampling should be very small.
Percentage weight of tungstic acid (WO3) in wolfram ores as determined by:
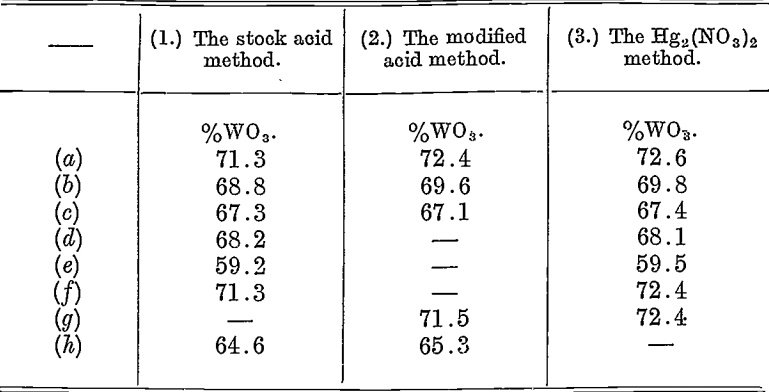
It will be seen that Tungsten method Assaying (1) tends to give the lowest Results, which occasionally are below those of methods (2) and (3) by more than desirable limits, which may be placed at 0.6% WO3 on this class of material, e.g.—(a), (b), and (f). Assay Method (2) agrees well with method (3), but the latter method inclines to give the higher results. It need only be stated, in conclusion, that the above results represent average results obtained in the writer’s practice.
