Table of Contents
Many mineral samples are sent to Government agencies with the request that they be assayed or chemically analyzed. It should be emphasized that there are no Federal agencies, except for the U.S. Mints, where assays and quantitative analyses are made for the public.
Many of the States maintain a Bureau of Mines, a Geological Survey, or some similar organization, frequently at a State university, where minerals found by residents of the State will be identified free of charge. Usually, a charge is made for assays. Specific tests, as on clays, are made by some of these offices.
The Federal Bureau of Mines , although it attempts to avoid duplication of services rendered by State agencies, is authorized to give advice regarding prospective markets. It does not compete with private assayers and chemists, but it will accept samples sent to its field laboratories, and identify them by visual or microscopic inspection. This examination is usually sufficient to indicate whether the material has commercial value, or at least if the expense of an assay would be warranted. Except in connection with its own technical investigations, no assays or other special tests are made by the Bureau of Mines. A general statement of policy regarding the Bureau’s mineralogical services that are available to the public is included in the Federal Register.
The U.S. Geological Survey and the Smithsonian Institution also make mineral identifications of specimens as a public service. However, neither of these agencies nor the Bureau of Mines will provide such services on long suites of specimens submitted by mineral dealers, well drillers, or others, when such work can be done regularly by commercial concerns.
The U.S. Mint, Denver, Colo., provides assays of ores for gold, silver, copper, lead, and zinc. The U.S. Mints also perform assays of gold and silver bullion. The special services provided, the charges involved, and the locations of U.S. Mints are presented in the Department of Treasury publication, Title 31-Money and Finance: Treasury. A copy of this publication can be obtained from the Bureau of Mint, U.S. Treasury Department, Washington, D.C. 20402.
Procedure for Obtaining Ore Samples
The economic exploitation of a mineral deposit follows a more or less set sequence. Invariably, the first step is identification to determine whether the ore to be mined or the mineral to be sold is actually what it is thought to be. An identification is a qualitative examination designed to give information as to what a substance may be rather than how much of it may be present.
If the identification shows the material to be one of possible commercial value, the next step is to take a sample representative of the exposed face or outcrop for assay or quantitative chemical analysis. Preliminary consideration also should be given to certain economic aspects. For example, no mineral deposit, irrespective of its size and grade, is of immediate value if it is so inaccessible that the product cannot be mined, processed, transported, and sold at a profit on a competitive market.
Sampling should always be made on a fresh surface. There are two suggested ways of proceeding, of which the second is the more reliable.
First method: With a hammer, knock off chips of approximately equal size at regular intervals over the whole width of the mineral occurrence.
Second method: Draw two parallel lines 3 inches apart, marking the width to be sampled. With a cold chisel, or a moil of tempered steel, cut out a groove, one-half inch or so in depth, for the whole length between the two lines. The material thus chiseled out will constitute the sample. It is very important to carefully collect the whole of the detached material, both chips and dust, on a sheet of canvas, strong cotton, or cloth.
A sample thus obtained will be fairly representative of the mineralization at the place where it was taken. In order to make an estimate of the value of a mineral deposit, it will be necessary to repeat the sampling process at regular intervals, about every 20 feet, and at the same time, take note of the location and the length of the grooves thus made. Bag and number each sample separately.
If the analyses of samples taken in this manner show promise, the succeeding steps in the procedure are (1) a thorough sampling of the deposit to determine grade and tonnage available, (2) beneficiation or smelting tests, (3) a marketing survey, and (4) consideration of such engineering factors as mining methods, design of concentration or treatment plant, water and power supply, and estimates of costs.
These operations are usually difficult and expensive and should be carried out under the guidance of trained engineers. The development of a prospect from the original discovery to a producing mine is a major undertaking that requires considerable capital and experience.
Advice as to how to proceed with a project may be obtained from a number of sources. Advice of a general sort, requiring no field work or laboratory testing, can be obtained from the Bureau of Mines. (See appendix A.) For projects where a paid, professional consultant is needed, names and addresses may be found in the advertising sections of appropriate trade journals. The Directory of Members of the American Institute of Mining and Metallurgical Engineers, 345 East 47th Street, New York 10017, lists the names and addresses of mining engineers and metallurgists available for consultation. For names of consultants specializing in metallurgical and chemical problems, a useful source is the classified directory of the Association of Consulting Chemists & Chemical Engineers, 50 East 41st Street, New York.
Procedure for Sampling Bullion and Scrap
The proper sampling of bullion and/or scrap precious metals, such as electronic, dental, or jewelry scrap, is very difficult and requires the utmost precautions. According to Bugbee, the principal problem encountered in sampling bars or ingots involves the irregular distribution of the various constituents caused by segregation when the bar freezes from the molten state. When a bar or ingot is poured, solidification begins first at the cooler walls of the mold, and the constituent having the highest melting point solidifies first. The material that freezes last, near the upper middle of the bar, is therefore enriched in the lowest melting point metal.
Obviously, the best way to sample material of this type would be while it is molten and homogeneous. Since this is normally impossible except to the refiner, drilling or sawing the bar offer the best alternatives. Sawing is more laborious and destroys the continuity of the bar since it would have to be sawed completely through. In drilling the bar, it is important to sample both the ends as well as the middle and to drill either completely through the bar or to drill half way through from both sides. The material removed from the bar must, in turn, be carefully sampled for assay or, in some cases, remelted and either sampled while molten or frozen rapidly by pouring into water so that segregation does not occur. Proper sampling of obviously heterogeneous metals such as industrial, jewelry, or dental scrap requires that the material be melted prior to sampling. The sampling of scrap electronic parts consisting of circuit boards, and components such as transistors and integrated circuits, is a difficult task and requires an assay on each constituent and an estimate on their total number. It may be feasible in some cases, such as in sampling circuit boards, to roast the sample to remove the organic material and then analyze the ash for the metal values of interest.
Description of Testing Techniques
Various testing techniques are available to the public at commercial laboratories, the choice of which depends on the problem to be solved. The most useful techniques are described to familiarise the reader with their application as well as their limitations.
Chemical Analysis
A chemical analysis is often used to determine how much of a particular element is present in a sample. Chemical analyses are normally very precise and accurate, but they are often less sensitive and more time consuming than instrumental analyses. It is because of these disadvantages that wet chemical techniques are usually not suitable for the determination of the noble metals (gold, silver, platinum-group metals) in ores and concentrates. In recent years, there has been a merging and combining of chemical and instrumental techniques to take advantage of the best features of both approaches. For example, an ore sample might be put into solution and separated chemically into fractions that are subsequently analyzed for selected elements by either standard chemical or atomic absorption procedures.
The value of a quantitative analysis depends on how representative the sample is of the total bulk of material to be tested. No ore or mineral deposit is uniform throughout; consequently, an analysis of a sample consisting of a single specimen or a few randomly chosen pieces of rock is useless in attempting to evaluate a potential deposit. This information can be obtained only by prescribed scientific methods of sampling.
Fire Assay Analysis
The term “fire assaying” is applied to a quantitative determination procedure in which a precious metal is separated from impurities by a fusion process and weighed to determine the amount of that metal present in the original sample. This method is normally used for the determination of gold and silver in ores, concentrates, and in various metal alloys. It can also be used in conjunction with atomic absorption and spectrographs procedures for the determination of platinum and the platinum-group metals. Excellent texts have been written on fire assaying by Bugbee and Shepard and Dietrich.
In general, the procedure involves the addition of various fluxing materials to the ore or sample, which when heated to about 1,900° F form a readily fusible homogeneous slag. Concurrent with the slag formation, a collecting or alloying metal, usually lead, is produced in the molten mass by reduction of part of the slag mixture. The noble metals are reduced from the mass and simultaneously collected by the droplets and mist of falling lead, forming a pool at the bottom of the slag. The molten mix is poured into an iron mold, and after cooling, the lead bullion containing the noble metals is physically separated from the glasslike slag and treated by a process called cupellation. This separates the lead from the precious metals by oxidizing the lead, which is absorbed into a special bone-ash dish called a cupel. The precious metals are left as a small bead on the surface of the cupel. In an analysis for gold and silver, the bead is weighed on a special balance that gives the combined weight of gold and silver in the sample. The bead is then treated with dilute nitric acid, which dissolves the silver but does not put any of the gold into solution. The bead is then reweighed to determine its gold content. In the analysis of platinum or the platinum-group metals, usually a small, known amount of silver is added before the fusion process. The resulting bead after cupellation is then analyzed by either spectrographic or atomic absorption methods.
Although there are many advantages to the use of fire assay, probably the most important is that there are no ores, concentrates, or alloys that cannot be analyzed by this method if it is properly performed. Furthermore, large and, consequently, more representative samples may be analyzed. In addition, the procedure has excellent sensitivity, less than 0.005 oz/ton of gold may be determined, and the method is specific for the noble metals. The detection limit for each of the platinum-group metals is about 0.001 oz/ton, based on the analysis of an assay ton (29.2 grams) by a fire-assay-spectrographic procedure. The disadvantages are that the technique is normally applied only to the noble metals. It requires more time and therefore is more costly than some other procedures. It also requires special balances and furnaces not normally found in a chemical laboratory.
Optical Emission Spectrographic Analysis
This analysis technique is based upon the principle that when a sample is heated to high temperature in an electrical arc, causing the sample to be volatilized completely, each element present in the sample emits a unique spectra that can be used to identify that particular element. In usual practice, a very small amount (5 to 50 mg) of finely pulverized ore sample (or filings if the sample is metallic) is vaporized completely, and the resulting spectra is recorded and used to determine the metals present. This general procedure can also be used to estimate the approximate concentration of each metal detected. If necessary, this technique can be further specialized to permit the quantitative determination of selected elements. The chief advantage of a spectrographs analysis is that 30 to 50 elements can be readily determined at quite low concentration levels. Consequently, a large number of samples can be surveyed rapidly to determine their general elemental composition. An important disadvantage is that most of the noble metals cannot normally be detected below about 30 parts per million (about 1 oz/ton) in ores and concentrates. Another disadvantage in applying this technique for detecting precious metals at low concentrations is the possibility of misidentification due to interferences from other elements in the sample. Since the usual requirement is to detect noble metals at concentration levels as low as 0.01 oz/ton, the spectrographic approach is not recommended. More specific information on spectrographic analysis may be gained from a good text such as the one by Aherns and Taylor.
X-Ray Analysis
In X-ray fluorescence analysis, the sample is exposed to a high-intensity gamma ray or X-ray beam, which causes each element present in the sample to emit characteristic X-rays that can be used to identify the respective elements. The intensity of this secondary X-radiation from the sample is also directly proportional to the concentration of each element that is present. In ordinary practice, a small amount of finely pulverized sample is placed in the X-ray instrument and excited by the X-ray beam. The whole range of emitted X-rays can be recorded to determine which elements are present or X-rays emitted from a particular element can be counted and compared with those of known standards to determine the exact concentration. The main advantages of this X-ray fluorescence technique are that it is simple rapid, highly reliable, and quite accurate providing proper calibration has been achieved for each type of sample being analyzed. Some disadvantages are that this technique is matrix-dependent and, consequently, it cannot be used to determine the metal content of ores, concentrates, or alloys without first being carefully calibrated to handle each particular type of material. Another disadvantage is that most of the noble metals cannot be detected below about 50 to 100 parts per million (2 to 3 oz/ton). Therefore, this approach is not recommended for the direct determination of gold, silver, or the platinum-group metals in ores or concentrates. Liebhafsky, Pfeiffer, Winslow, and Zemany give an excellent discussion on the application of X-ray fluorescence analysis techniques.
Another useful tool is X-ray diffraction. This technique is based upon the fact that the crystal structure of any given sample causes the incident X-rays to be diffracted in a manner characteristic of that particular material. The resulting X-ray diffraction pattern is like a fingerprint for that material, and can be used to identify it even in the presence of other crystalline materials. Over 20,000 different minerals, metals, and compounds have been catalogued by their characteristic X-ray diffraction patterns.
The use of this catalogue simplifies the identification of the various components in any crystalline sample. The advantage of X-ray diffraction is that it tells something about the composition and structure. Ordinarily, X-ray diffraction is not used to make quantitative determinations. However, in special cases, such as the determination of free silica in a coal mine dust sample, it is feasible to make quantitative analyses.
Atomic Absorption Analysis
Atomic absorption is based on the fact that a free atom is capable of absorbing light of the same wavelength it would normally emit. If light emitted by an element inside a special lamp is passed through a gaseous cloud containing this element in the atomic state, then the atoms from this element, and only this element, will absorb this light. In practice, the gaseous cloud is formed by aspirating a solution of the sample to be analyzed into a flame of sufficient temperature to reduce the element to its atomic state. The absorbance of the light from the special lamp by the aspirated sample solution is then compared with the absorbances of suitable standards analyzed in a similar manner. Prior to analysis, the sample obviously must be put into solution with suitable solvents. In gold analysis, this can be accomplished readily with aqua regia , a mixture of nitric and hydrochloric acids. For the analysis of gold and the platinum-group metals, dissolution and direct analysis of ores or concentrates is impractical; the very small absorption signal from the low levels of these metals that are normally encountered is masked by light scattering in the flame caused by high concentrations of dissolved solids. Consequently, it is necessary to resort to methods involving organic extraction of the precious metals from the acid solution and analysis of these extracts, or the use of fire assay to preconcentrate the precious metals into a small bead that can be dissolved and analyzed by atomic absorption. Using an acid digestion-organic extraction method on a 1-gram sample, less than 0.01 ounce of gold per ton of ore may be measured. If a fire assay preconcentration is used, the sensitivity can be extended still further. Atomic absorption analysis, when properly applied, has few disadvantages, and it is fast, accurate, and economical. It has the advantage that the equipment is comparatively inexpensive. Furthermore, since the method may be employed for the analysis of most metals, many testing laboratories use this technique. An excellent and comprehensive text on this type of analysis is the one by Ramirez-Munoz.
Neutron Activation Analysis
This method of analysis is based on the principle that when a sample is subjected to bombardment by neutrons, some of the stable atoms that make up the sample will absorb neutrons and become radioactive. These radioactive atoms will, in turn, emit gamma rays the energies of which are characteristic of the particular elements. Using suitable and rather sophisticated electronic counting equipment, these gamma rays can be detected, and the elements and amounts present in the sample can be determined. Alternatively, to obtain greater sensitivity and avoid interferences, the activated sample may be dissolved and the elements of interest chemically separated prior to being counted. For gold and silver, the direct electronic method has excellent sensitivity, but for the platinum-group metals, chemical separation techniques are required. This type of analysis would not normally be utilized for ores or concentrates. Analysis of the noble metals by this method is usually limited to rather esoteric samples, such as moon rocks, filings from ancient coins, and geological samples with exceedingly small and noneconomic amounts of metals present. Only small samples arc usually irradiated in nuclear reactors, and consequently any material that is not clearly homogeneous, such as a gold ore, can give erroneous results. Furthermore, the method is expensive and is only performed commercially in a few laboratories.
Bullion Assay
The analysis of gold or silver bullion is more complex, time consuming, and expensive than a standard fire assay. This is readily understandable since the material the sample represents is very valuable and, therefore, the analysis warrants the utmost attention. Great care must be exercised in obtaining proper samples that are truly representative of the whole material. The assay of gold and silver bullion is expressed in parts per thousand called fineness. For example, a gold bullion containing 99 percent gold, or 990 parts per thousand, is 990 fine.
The assay for silver in silver bullion or gold bullion may be accomplished either by cupellation or by wet chemical volumetric methods. The cupellation method is subject to more errors, and if accurate values are needed, as in the assay of fine silver bullion, the chemical procedure must be applied. The errors in the cupellation assay for silver are corrected by means of a check or proof assay of a synthetic sample of approximately the same weight and composition as the bullion sample. The check and bullion samples are cupelled side by side, and it is assumed that the losses as determined for the check sample also apply to the bullion. Therefore, the method involves a preliminary assay to determine the approximate composition of the bullion before the check assay can be prepared. The sample size normally taken is about one-half gram. The assay of fine silver bullion, greater than 990 fine, is normally accomplished by a wet chemical procedure called the “Gay Lussac-U.S. Mint Method”. In this technique, the silver sample, about 1 gram, is accurately weighed, dissolved in nitric acid, and about 99.8 percent of the silver is precipitated with a 100-ml NaCl solution that has been previously standardized with pure silver. Subsequent milliliter additions of the diluted standardized NaCl solution are added until no further precipitation occurs. A more recent refinement of this method uses atomic absorption to determine the small amount of silver remaining In solution after the initial precipitation with the standardized NaCl solution.
The general method of assaying gold bullion is by cupellation and parting accompanied by check or proof assays on synthetic alloys corresponding to the composition and weight of the bullion. Therefore, the method usually requires a preliminary assay to obtain an approximate analysis. As in the case of silver bullion analysis , the check and bullion samples are cupelled side by side, and any loss or gain in the check sample, which is called the surcharge, is applied to the bullion sample. In the parting process, which separates the silver from the gold, the silver-gold ratio must be kept between 2 to 4 parts silver to 1 part gold, otherwise proper parting will not be achieved. Consequently, silver must normally be added to both the bullion and proof sample. The resulting bead from the cupellation must be rolled very thin and and parting procedure must be carefully standardized. In both the silver and gold bullion assay, results are obtained on duplicate samples.
Prices and Fees
The trade generally recognizes three qualities of work. The first and lowest priced analysis is designated by such terms as routine, engineer’s survey, and preliminary. A higher priced, more careful analysis is called control, and the third and most expensive is called umpire. Unfortunately, there is no way of setting up standards of quality on the basis of accuracy because some elements, such as iron or copper, can be determined in the usual samples with little effort to a high degree of accuracy, whereas others require time-consuming procedures and great skill, in spite of which accuracy of results is only fair.
The price ranges listed are typical of the prices being quoted by commercial laboratories as of January 1, 1976, for routine analyses. The listed values are for single, finely ground samples. An extra charge of $1 to $5 per sample is assessed if the sample has to be prepared for analysis. Discounts are usually granted if many samples of the same type are submitted at one time.
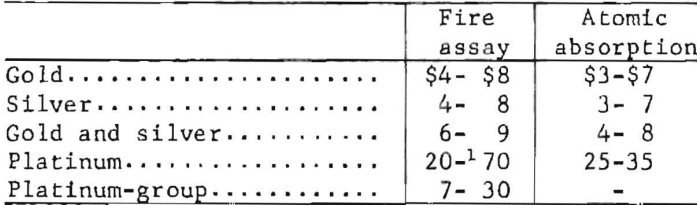
Higher prices are charged for the assay of the richer samples, such as bullion, sweepings, and jewelry. Control and umpire analyses cost two to three times the price of routine analyses.
A qualitative spectrographic analysis to determine the general elemental composition costs about $15 to $30 per sample. An X-ray diffraction analysis to determine the mineral composition of a sample costs about $30 to $50.
Most of the common metals in ores, concentrates, and alloys can be determined with reasonable accuracy by atomic absorption. The cost is $1.50 to $3 for the first element, plus about $1 to $2 for each succeeding element in the same sample. Some of the metals that can be determined by this technique are bismuth, cadmium, chromium, cobalt, copper, iron, lead, manganese, molybdenum, nickel, tin, and zinc.
A few laboratories perform mineralogical services, the cost of which varies with the type of analytical work required. Typical services and estimated costs are as follows:
Petrographic and mineralogical examination per hour………………………………………..$10
Thin sections………………………………………………………………………………………………….$4-14
Polished sections……………………………………………………………………………………………….4-5
Grain mounts…………………………………………………………………………………………………….4-10
Particle size distribution…………………………………………………………………………………….25-35
Specific gravity…………………………………………………………………………………………………..4-6

