Table of Contents
A semi-quantitative test for the presence and identification of cesium and rubidium in rocks, clays, and mineral waters has been developed by the Bureau of Mines. The test can be used in the field, as a guide for the prospector, geologist, or mining engineer engaged in location or development work. It comprises two simple spot tests of a solution prepared from the mineral sample. Phosphomolybdic acid, added to a drop of the test solution, detects cesium, rubidium, and potassium in quantities as low as 60 parts per million. Silicomolybdic acid, added to a second drop of test solution, enables the operator to distinguish between cesium and/or rubidium and potassium. Test procedures, equipment requirements, and limitations of the method are described in this report.
Cesium and rubidium were discovered in 1860 and 1861 by Bunsen and Kirchhoff with a spectroscope. The elements usually occur together, particularly in granite and granitic pegmatites. Rubidium does not occur as an essential constituent of any known mineral, yet it is found as a minor element in so many minerals that rubidium is 16th in abundance in the earth’s crust. Cesium, which ranks as the 37th most abundant element, is the principal component of pollucite and a minor element in a wide variety of minerals.
Currently the two elements have only limited industrial use but hold considerable promise for the future. Before 1957 cesium was recovered from pollucite, but at present most of the limited domestic production of both cesium and rubidium results from retreating residues isolated during the extraction of lithium compounds from lepidolite. Domestic consumption of these elements and their compounds increased in the past few years from a few hundred to a few thousand pounds annually. Most of the increase was due to enlarged activity and usage in research directed to the development of new, large-scale uses.
At present the two elements find their principal usage in infrared lamps, telescopes, binoculars, and spectrometers; in photoelectric cells; vacuum tubes; scintillation counters; frequency and time standards; medicines; and in ceramics. Possible uses of the future may include utilization as ion-type rocket-engine fuels for interplanetary travel, as plasmas in thermionic converters to change heat to electricity, and as a heat transfer medium in nuclear power systems.
In the event some of these larger scale uses for cesium and rubidium come to fruition, new raw material sources must be located, and more efficient and lower cost recovery and processing methods must be developed. Inasmuch as the elements usually occur in combination with other more common elements, and rarely exhibit distinctive visual characteristics, it is anticipated that a simple and reliable field test enabling detection of their presence will greatly facilitate the search for new deposits.
Occurrence of Cesium and Rubidium
Cesium and rubidium usually occur together as minor constituents of a wide variety of minerals. As much as 3.5 percent of either element may be present in such minerals as rhodonite, triphylite, lithiophylite, lepidolite, carnallite, beryl, leucite, spodumene, petalite, zinnwaldite, micas, and potash feldspars. Granitic rocks generally contain a few hundredths of a percent of rubidium and somewhat less cesium, presumably associated with the potash feldspar in this type of rock. Cesium and rubidium also are found in mineral springs, brines, bitterns, saline waters, and saline deposits. Average sea water contains 0.2 milligram of cesium and 2.0 milligrams of rubidium per liter.
Pollucite, a hydrated cesium aluminum silicate, is the only known mineral that contains cesium as an essential and major constituent. The mineral may contain up to 42.5 percent Cs2O, but usually averages about 25 percent. Rubidium is often present in pollucite in quantities as high as 3 percent Rb2O. Potentially important deposits of the mineral have been found in Canada and Africa, and minor tonnages of pollucite-bearing rocks have been mined from deposits in South Dakota and Maine. As pollucite and quartz are difficult to distinguish from one another in the field, it is quite possible, if not probable, that many deposits remain to be discovered.
Evaluation of Known Methods
A survey of the literature revealed that many methods have been suggested for detecting rubidium and cesium in diverse types of materials. As the objective of the investigation was the development of a reliable test for field use, only the more simple methods warranted evaluation. Therefore, initial studies were limited to determinations of the reliability and sensitivity of flame, bead, microchemical, and spot tests for detecting and/or distinguishing rubidium and cesium in standard samples.
Flame and Bead Tests
As rubidium and cesium were discovered by their distinctive flame spectrum, flame coloration tests were studied first as a possible means of detecting and/or distinguishing between these elements. It was ascertained that rubidium or cesium could be detected by this method only in relatively rich materials essentially free of one of the two elements and of potassium, which interfered with the test. As such field occurrences are rare, flame tests were deemed impractical.
The possibility of employing bead tests for field identification of the elements also was explored. Pulverized samples, containing known amounts of rubidium and cesium, were mixed with borax, microcosmic salt, sodium fluoride, and/or other fluxes and melted in (1) oxidizing or (2) reducing flames. None of the many beads prepared and examined both in hot and cold states had a distinctive color that could be related to the rubidium or cesium content of the samples.
Microchemical Tests
Microchemical tests, based on the use of various inorganic and/or organic compounds that might form characteristic microcrystals with either rubidium or cesium, were investigated as methods possibly applicable to field use. Of many procedures mentioned in the literature, eight of the least complicated were tested, using solutions containing different concentrations of rubidium and cesium. Three of the methods tested had some merit for detecting rubidium and cesium.
Dipicrylamine and 2,4-dinitrophenol precipitated easily identified crystals from solutions containing low concentrations of rubidium and cesium. These organic chemicals, however, were not selective, in that both rubidium and cesium were precipitated in similar crystal form. When potassium was present, it too was precipitated as crystals that were difficult to distinguish from those formed by rubidium or cesium.
A dilute acetic acid solution of potassium bismuth iodide was the only chemical tested that was selective for cesium. The addition of this chemical to an unknown solution formed relatively large red hexagonal crystals within 30 minutes if as little as 0.1 gram of cesium was present per liter of solution. Thallium and ammonium ions interfered with the test, and the presence of potassium and rubidium reduced the sensitivity.
All the microchemical tests evaluated proved to have serious limitations when used to detect rubidium and cesium. Generally, the test conditions required careful control to promote the formation of distinctive crystals. Several elements, among them potassium, an element commonly associated with rubidium and cesium, interfered with the reliability and sensitivity of the tests. None of the tests was selective for rubidium, and only the potassium bismuth iodide test was selective for cesium.
Chemical Spot Tests
As the flame, bead, and microchemical methods studied appeared unsuited to field use for detecting rubidium and cesium, attention was directed to an investigation of spot test methods. Of the many chemicals known to form precipitates with rubidium and cesium, 32 were selected for preliminary testing to define their relative sensitivity and selectivity. Only phosphomolybdic, phosphotungstic, silicomolybdic, and silicotungstic acids were deemed sufficiently promising for more detailed investigation of their possible use in the field.
The selectivities and sensitivities of these four chemicals were tested, using standard solutions containing different concentrations of rubidium, cesium, potassium, and sodium as chlorides. A series of drops of standard solution was placed on black spot test paper. Drops of solutions of the four chemicals were added to individual drops of the solutions tested, and the nature of the precipitates formed noted. This study demonstrated that the phospho-acids were more sensitive than the silico-acids for detecting small concentrations of rubidium and cesium. However, the phospho-acids, unfortunately, formed precipitates with potassium. Conversely, the silico-acids, while not so sensitive, were somewhat more selective as they precipitated only rubidium and cesium, if the potassium concentration was lower than 100 grams per liter of solution.
Additional testing determined that the phospho- and silicomolybdic acids were more sensitive than the corresponding tungstic acids and that the molybdic acids were less affected by possible interfering elements. Sodium, lithium, thallium, and ammonium were the main interfering ions, but only the ammonium ion must be strictly avoided when using phospho- or silicomolybdic acids. Sodium and lithium did not interfere unless these elements were present in exceptionally high amounts. Iron salts tended to color the spot tests a green color but did not obscure the alkali precipitate.
The most reliable results were obtained when using 20 percent, by weight, water solutions of phosphomolybdic acid and saturated water solutions of silicomolybdic acid. Several samples of supposedly chemically pure silicomolybdic acid, used in the research, varied considerably in water solubility, presumably because the reagent deteriorates. For this reason it is recommended that the saturated solution of this reagent be prepared in the following manner: Add 20 percent by weight of silicomolybdic or phosphomolybdic acid to distilled water. Agitate the mixture for a few minutes, and allow the solution and reagent to stand in contact with one another for 2 hours. Any excess reagent then should be filtered from the saturated solution and discarded. The phosphomolybdic and silicomolybdic acid solutions, if prepared in the manner described, are stable.
Extraction of Rubidium and Cesium from Raw Materials
After determining that spot tests would detect rubidium and cesium, attention was turned to the problem of devising a relatively simple method of extracting these elements from typical raw materials and preparing solutions suitable for spot testing. A number of acid digestion and fusion methods were tested on samples of rubidium and cesium-bearing feldspars, granites, clays, and micas; and pollucite, lepidolite, pegmatite, carnallite, and spodumene ores.
Of the different methods tried, two were simple and reasonably effective for extracting rubidium and cesium from typical raw materials and, therefore, adaptable to field use. Digestion of a pulverized sample of ore with hot hydrochloric acid was the simpler of the two methods. This method satisfactorily dissolved cesium from all raw materials examined. However, on a few samples, only a small part of the contained rubidium was dissolved by hot hydrochloric acid.
The second method involved a fusion of a powdered sample admixed with a flux of calcium carbonate and calcium chloride, followed by water extraction of the cesium and rubidium chlorides formed. This method, although more complicated than the acid digestion procedure, was preferred, as it dependably extracted both cesium and rubidium from all raw materials examined. In both methods a minimum amount of solvent (acid or water) must be used, to insure that a concentrated solution is obtained for spot testing. If excess volumes of solvent are used, the solutions must be evaporated to eliminate diluting acid or water.
Field Test for Cesium and Rubidium
The field test comprises extraction of rubidium and cesium from samples by either acid or fusion methods. The solution thus prepared is spot tested first with phosphomolybdic acid and then with silicornolybdic acid. The presence and approximate amounts of cesium or rubidium in the solution are determined by observing the color, nature, and time required to form characteristic precipitates. The detailed test procedure recommended is described as follows.
Sample Preparation
The sample selected for examination should be dried, crushed into fine fragments, and 5 to 10 grams pulverized to pass 200 mesh, in a mortar and pestle. The pulverized sample should be screened on a 200-mesh sieve, because cesium and rubidium are only slowly dissolved from coarse material by either of the two extraction methods.
Dissolution of Cesium and Rubidium
A solution suitable for spot tests can be prepared by either of two methods. The first and simpler method comprises placing about 5 grams (1 level teaspoon) of pulverized material in a porcelain crucible, adding and mixing 10 milliliters (2 level teaspoons) of concentrated hydrochloric acid and then gently heating the mixture for 10 minutes to incipient boiling, being careful not to boil away all the acid. The solution then is poured through a No. 40-series Whatman filter paper without using additional water. The clear solution, when cool, is ready for use in the spot test.
The second method, which is more complicated but assures dissolution of rubidium, comprises fusion of the sample with a flux. About 5 grams of pulverized material is thoroughly mixed with 10 grams of powdered calcium carbonate and 2 grams of powdered calcium chloride. The mixture is placed in a No. 2 porcelain crucible and the covered crucible heated for 30 or more minutes at a red heat. After cooling, the fused mass is transferred to the mortar and reground through 200 mesh. The pulverized material then is placed in a clean porcelain crucible and mixed with small portions of water until thoroughly wetted. Another 5 milliliters of water then is added and the crucible heated gently for about 5 minutes while occasionally stirring the mixture. The contents of the crucible are filtered, and the clean solution recovered is reserved for spot testing.
Spot Testing
The spot tests, by means of which the presence of rubidium and/or cesium can be detected, are simple to perform. A drop of the solution prepared by either of the methods described is placed on a sheet of glossy black spot-test paper, using a clean medicine dropper. A drop of 20 percent phosphomolybdic acid is added. If a yellow precipitate does not form immediately, neither cesium nor rubidium is present. If a precipitate forms, it indicates (1) the presence in the solution of small to large amounts of cesium plus rubidium, depending on the nature of the precipitate and the time lapse before the precipitate forms; or (2) it reveals the presence of over 5 grams of potassium per liter of solution.
If the test with phosphomolybdic acid is positive, a second spot test is made by adding a drop of saturated silicomolybdic acid to a fresh drop of solution. As silicomolybdic acid does not precipitate potassium and reacts only slowly with large amounts of rubidium, formation of a precipitate in a reasonable length of time indicates the presence of cesium. Table 1 was prepared as a convenient reference for interpreting the results of the spot tests with the two reagents.
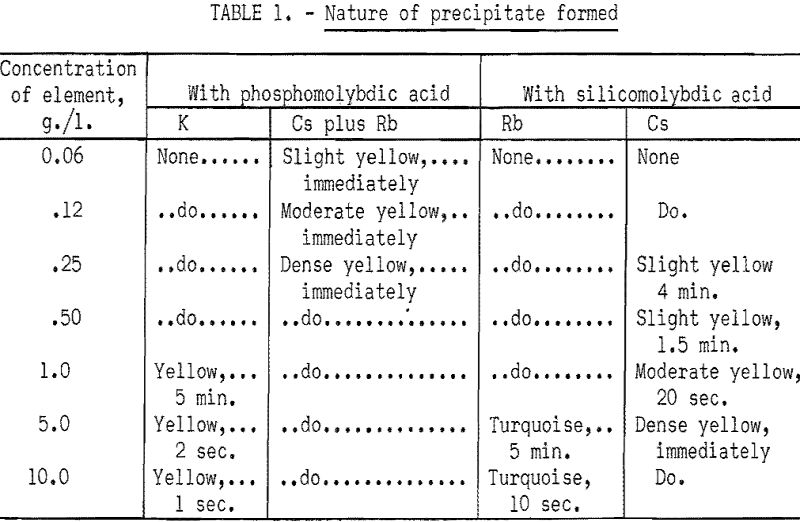
Semiquantitative estimates may be obtained by diluting reagents and timing the appearance of a precipitate in unknown solutions and comparing the reactions with those obtained on solutions containing known amounts of potassium, rubidium, or cesium. As an example, a 5 percent solution of phosphomolybdic acid will not show potassium under 4 grams per liter in less than a minute, but will show a yellow precipitate with 0.15 gram of cesium per liter immediately or 0.15 gram of rubidium per liter in ½ minute. This technique would eliminate interference of less than 4 grams per liter of potassium in the test for rubidium and cesium. A variation of this test would be to dilute a sample drop five times with distilled water and test with 5 percent phosphomolybdic acid. The formation of a yellow precipitate then would indicate the presence of rubidium or cesium or of 20 grams of potassium per liter, which is not likely from most natural sources. Similar variations of the standard test procedure with the silicomolybdic acid reagent are possible to obtain semiquantitative analysis for cesium.
Chemicals and Equipment Required
The suggested chemicals and equipment for the field test listed are obtainable from laboratory supply companies. Plastic bottles are recommended as containers for reagents.
Chemicals
- Phosphomolybdic acid (20 percent solution) prepared from c.p. reagent.
- Silicomolybdic acid (saturated solution) prepared from c.p. reagent.
- Hydrochloric acid (concentrated).
- Calcium carbonate or calcium oxide (powdered).
- Calcium chloride (powdered and tightly stoppered to prevent absorption of moisture from the air).
- Distilled water.
Equipment
- Porcelain crucibles – sizes 1, 1A, and 2 with lid for size 2.
- Mortar and pestle – size 0 or larger.
- Any type of burner that can be used in the field and is capable of producing a flame temperature of 900° C. or above.
- No. 40 Whatman filter paper or equivalent.
- Funnel.
- Glazed, black paper.
- Safety matches.
- Crucible tongs.
- Medicine droppers (3).
- Plastic or stainless steel teaspoon.
- A 3-inch diameter 200-mesh sieve.
