The Bureau of the Mint of the United States Treasury maintains offices for the purchase of gold-bullion, and this paper describes an investigation to establish the reasonable differences in the assay-results at the various institutions which may be commercially allowable in the settlements between them. Beginning with the comparative assay of proof-gold at the Philadelphia mint and the Utrecht mint, which shows 0.00002 as the closest agreement now possible, nine tables of comparative results, taken from the regular work of the service, are given. These tables begin with very fine gold, produced in an electrolytic refinery, showing close agreement in the assay-results, and follow through decreasing gold fineness and increasing amounts and complexity of base metals to very impure and complex bars produced at cyanide-mills, some of which give widely-varying results. Next is given a series of results on samples, prepared and sent out to various laboratories in the service, to test the influence of different metals and various combinations upon the agreement of the assay-results; 11 samples were sent out and each one was assayed from 44 to 71 times, making a total of 623 assays. To these are added 107 assays of identical samples of coin-gold.
On a previous occasion, I have endeavored to show the degree of accuracy that may be expected in the ordinary everyday analysis of various materials, and on another occasion, have called attention to the accuracy of the commercial assay for silver. The present paper deals chiefly with an effort to establish certain commercial standards of agreement or accuracy in the assaying of gold-bullion for purchase in the various laboratories of the U. S. Mint Service.
Besides the coinage-mints at Philadelphia, New Orleans, and San Francisco, and the large assay-office on Wall street in New York City, all of which purchase and refine bullion; the Mint Bureau of the U. S. Treasury maintains eight smaller assay-offices, scattered about through the mining-districts of the country, which purchase bullion and ship it to the mints to be refined. These smaller offices were established as an aid to the mining industry of the country by giving the small miners the opportunity to sell their bullion easily and quickly for cash.
Owing to the particular and rigid methods of book-keeping of the Treasury Department, the mints are compelled to treat the bullion sent to them from the assay-offices in exactly the same manner as the bullion deposited directly by individuals, and the prices are carefully determined at which the assay- office bullion should be charged against the mint in the Treasury accounts. Naturally, discrepancies sometimes arise between the mints and the assay-offices. A very large proportion of these are small and are easily adjusted. In fact, most of them adjust themselves automatically, as they are on both sides of the account, and the gains and the losses over a period of time will counterbalance each other. On rare occasions, however, the differences require adjustment by umpire-assays in the laboratory of the Bureau of the Mint at Washington. For several years I have been gathering data upon the subject, and have had a series of assays made in order to establish standard limits of differences which might be considered as allowable on different classes of bullion.
The methods of assaying followed in the various institutions are substantially the same, and have grown up as the result of many years of experience, so that with careful work on pure bullion the results obtained at different institutions ought to agree very closely; but with impure bullion, that is, bullion containing other constituents besides gold and silver, the chances for variations in the results increase. The action of different impurities varies widely. Only small amounts of some impurities induce excessive variations in the results, while comparatively large amounts of others have but little effect, and, on the other hand, a combination of several impurities in a bullion may be most disastrous to any agreement of the assay- results.
The bullion is handled in the same manner at all the institutions. It is weighed as received, and then melted. Generally, a simple melting with soda or borax, or both, is sufficient, but sometimes it is more or less refined in the pot. In the case of large melts, 1,000 oz. or over, or of very impure bullion, a small sample may be dipped or poured out from the well-stirred pot and granulated in water. The granulations are used for the assay-sample. In general, however, the metal is cast into bars, and these bars are chipped, top and bottom, to obtain the assay-samples. The bars are again weighed and the assays made, when the value of the deposit is calculated from these data. If, however, the various assays made on a deposit do not agree well enough to satisfy the assayer, the bar is remelted, with or without refining in the pot.
The determination of gold in ores by the fire assay, when properly executed, is justly regarded as one of the most accurate of analytical methods. With ordinary care and an excellent bead-balance, 1 part of gold in more than 20,000,000 parts of ore can be readily and accurately determined. The determination of 1 part of gold in 5,000,000 parts of ore is very easily done. Until recently, however, it was rare for commercial ore-assaying to attain to the accuracy of 1 part in 5,000,000.
The ability to determine gold in ores with such great accuracy is due to the fact that very large amounts of ore, up to 0.25 kg., are taken for the assay, and on a high-grade button-balance the resulting bead can be weighed to 1/200 mg. In assaying bullion, however, such extreme accuracy is out of the question, for the simple reason that there is a limit to the amount of bullion that can be taken for the assay. To obtain the most accurate results the assay-sample must be weighed on the same high-grade balance on which is weighed the resulting cornet, and the sample also must be weighed with the same degree of care and accuracy as the cornet. Now, the load that a very sensitive bead-balance will safely carry is generally limited to 1 g., and the amount of metal generally taken for a gold-bullion assay is 0.5 g., or one-half of the maximum load of the balance.
Another point in bullion-assaying which militates against extreme accuracy in the results lies in the fact that the cornet which is weighed is itself gold, and, in high-grade bullions, it is a very large part of the sample taken for the assay, so that even slight errors in the handling of the cornet, resulting in slight losses or gains in its weight, count heavily against the highest accuracy of the results.
About two years ago samples of proof-gold were exchanged between the Philadelphia mint and the Utrecht mint, and these samples were assayed in comparison with the utmost care at both institutions, with the result that the Utrecht proof was pronounced slightly the better by both mints. The difference in the results of the assays at the two places was only 0.00002. This is by far the most careful and exhaustive comparison of gold-bullion assays known to me, and undoubtedly represents the limit of accuracy at present attained in such work.
Table I. shows a series of results obtained by three assayers working in the same laboratory upon fine gold from an electrolytic refinery. Each assayer worked upon the same sample in each set of assays as averaged, the samples being cut from both the top and the bottom of the bars. While there is a possibility that there may be some difference in composition between the top and bottom of the bars, yet in such high-grade material as this any such difference must be slight, and eight tests upon the subject showed a maximum difference between the top and bottom of only 0.0001, which is considerably less than many of the differences between individual assays. On the whole, then, the figures may be taken as fairly representing the ordinary run of commercial work upon such high-grade bullion. It will be noted that in several cases the figures exceed 1,000, which is due, in part at least, to the high grade of the material. It may also be due in part to the presence in this electrolytic gold of unusual impurities in very small amounts. These data emphasize the necessity of averaging a large number of assays to get a satisfactory determination of the fineness in such very high-grade material.
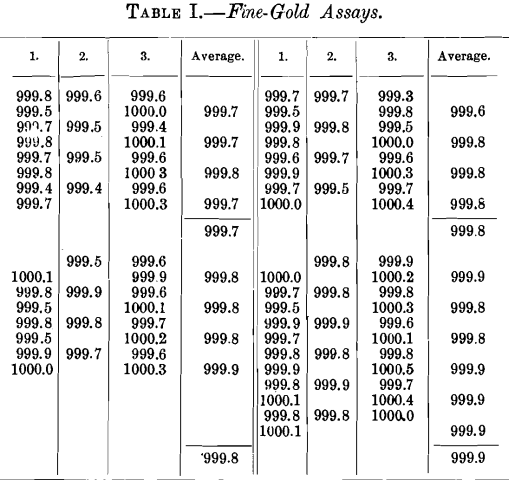
Table II. shows results obtained by various assayers in a single laboratory in assaying granulation-samples from a wide variety of bullion.
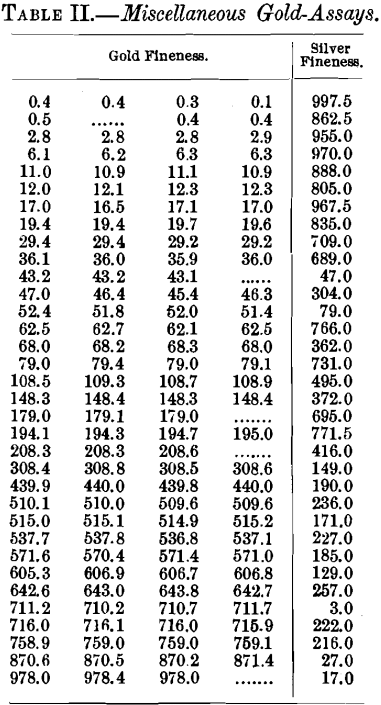
The figures given in Table III. are all taken from a single shipment and show the accuracy that can be obtained upon material of fairly uniform composition, being mostly gold and silver, with but little base metal present. This table shows, first, the results obtained at the assay-office where the bullion was originally purchased; and, second, the results obtained upon the same material when shipped to a mint. In some of these samples there is undoubtedly a difference between the tops and bottoms of the bars, but the figures show the agreement that may be expected between two institutions in arriving at the value of such deposits.
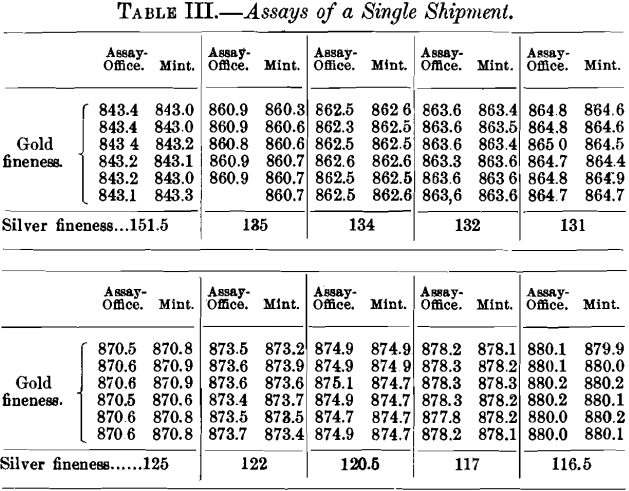
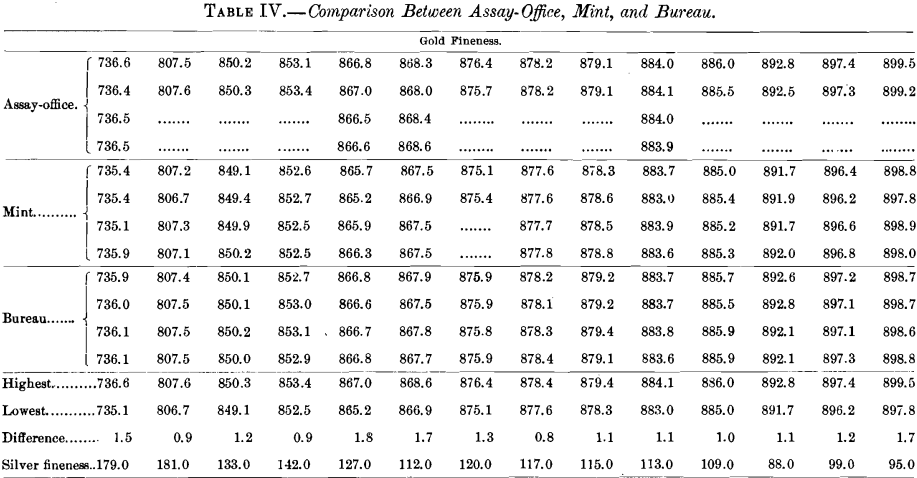
Table IV. gives the assays of 14 bars which were referred to the Bureau laboratory for adjustment, although the average differences between the mint and the assay-office were only slight.
The handling of bullion produced at mills using the cyanide-process of gold-extraction has given a great deal of trouble.
Even when properly prepared such bars are likely to be troublesome, but when, as not infrequently happens, the precipitates are not properly purified before being cast into bars they may give no end of trouble.
A very mild case of variation in cyanide-bars is shown in Table V. As received, these bars were chipped and the chips assayed. Since the figures thus obtained were considerably higher than the shipper’s figures, the bars were then carefully bored and the borings assayed. Finally, the bars were remelted, with small losses in each case, and granulations taken. The granulations were then assayed.
Table VI. exhibits the results obtained by sampling three cyanide-bars, high in gold and very low in silver, in three different ways. The assays show a wide variation on the chip- samples. While the drill-sample assays are fairly concordant for this class of material, the dip-sample assays agree much better and are to be preferred.
An assay-office had received a cyanide-bar which showed 546, 545.5, 546.2, 546 fine in gold. This was considered satisfactory, and it was shipped to a mint, but the chip-samples there yielded most varying results, as follows: 544.6, 535.2, 543, 535, 542.4, 555.6. The bar was then remelted, and granulations showed 550.2 and 551.2. Another cyanide-bar received at the same assay-office from the same mill showed 592, 593.9, 592.9, 593.3 fine in gold, and was accepted. It was shipped to the same mint, where chips showed 603.6 and 590, while borings showed 588 and 588.6. The bar, which weighed 559.65
oz. Troy, was remelted, with a loss of 1.78 oz., and granulations from the melt showed 601.8 and 601.8 fine in gold.
Having had a great deal of trouble with some bars from this mill, while others gave but little trouble, the assay-office gave one of the bad bars a very thorough treatment by melting and refining in the pot. As received, the bar weighed 643.30 oz. Troy, and was probably about 847 fine in gold. It was melted seven times, when it weighed 502.01 oz., showing a loss of 141.29 oz. in weight. The final bar was 933.4 fine in gold and 21 fine in silver. The gold-loss from this excessive course of meltings was only approximately 3.75 oz., most of which could undoubtedly be recovered from the slags.
The details of the meltings are shown in Table VII. It should be noted that the fourth melt shows practically no refining, and the weight was only slightly reduced, so that no practical change is shown in the assays.
From an extensive series of tests made at the San Francisco mint it was found that, as a rule, in the cyanide-bars from several California plants, the chip-samples taken from the outside of the bars would be about 2.5 fine less in gold than the borings when taken away from the edges of the bar, and that the borings gave satisfactory samples of the bars.
Thirteen miscellaneous deposits were united in a mass melt and cast into 17 bars weighing 2841.77 oz. Each bar was chipped twice, and each chip was assayed in duplicate for gold. Table VIII. shows the number of times the stated fineness was obtained :
The average of the 68 assays showed the mass to be 407.16 fine in gold.
When made from the highest grade of metals our coin-gold, 900 gold and 100 copper, does not segregate. The gold used may contain a very small amount of silver, but should be as free as possible from all other impurities, and the copper should be of the highest purity possible. Occasionally, in practice, however, there will be a slight segregation due to some impurities present in minute amounts. On one occasion an inside strip cut from a double eagle was assayed six times and yielded the following gradually decreasing figures: 900.2, 900.1, 899.9, 899.85, 899.5, 899.45. On another occasion a double eagle was cut as indicated in Fig. 1 and the following results were obtained:
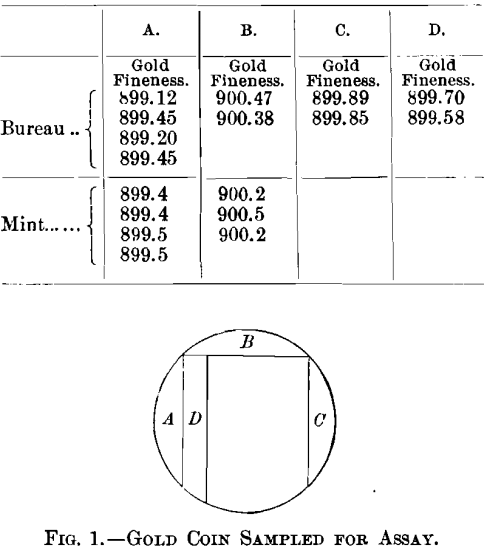
One of our most annoying and yet very interesting and instructive cases was a lot of foreign-coin gold, the product of a mint which is very careful in the manufacture of its coins; 12 deposits of this material were received at the Philadelphia mint from the New York assay-office. It was supposed to be 916 2/3 fine in gold, the balance being copper, and very uniform in composition, but the New York assays showed considerable variation. At Philadelphia one man assayed each deposit in duplicate, and he was checked by another man with a single assay, as shown in Table IX.

This bullion was diluted with copper to bring it down to the United States standard. While our standard is 900 fine, and the law allows a variation of one one-thousandth up or down, so that legally the coins may run from 899 to 901 fine in gold, yet the working-limits adopted at the mints are much narrower than the legal limits, and generally no gold ingots are passed by the assayer below 899.7 fine nor above 900.2 fine.
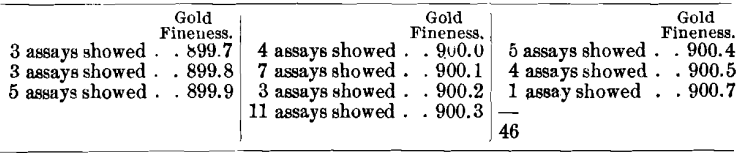
In making ingots from this metal an unusual number of melts had to be rejected and remelted for want of uniformity. It was expected that the coins made from this bullion would run low, but none of those regularly tested did. In fact, two from one delivery were most unusually high, viz.: 900.6 and 900.7. Thereupon 12 coins were selected from the same delivery and 46 assays were made upon them, with the following results:
The trouble with this metal undoubtedly arose from the presence of a small amount of some impurity causing a segregation of the gold, but enough work to decide what this was could not be given to the matter. In a similar case, with a different high-grade foreign-coin gold at the San Francisco mint, the trouble was traced to the presence of a minute amount of antimony.
In order to get a much wider range of comparison, and to test the influence of the different metals and of various combinations upon the gold assay, a series of samples was prepared in the Bureau laboratory and sent out to various laboratories in the service for assay. In preparing the samples the metal was thoroughly mixed by stirring when molten and remelted as often as appeared necessary. They were finally cast into small bars, and when sufficiently ductile were rolled out thin.
The strips were cut into small squares, and these were mixed up and the samples for each institution taken out of the mixed pile of pieces. In the case of the brittle bars, they were hammered out and rolled until they crumbled to pieces. The larger pieces were then cut up, and the whole mixed before the samples were taken out.
All through the preparation of the samples very great care was exercised, so that in each set every sample sent for assay should be identical, and thus eliminate from the assay-results all chances of differences being due to differences in the samples operated upon, and to confine the differences shown to the actual assay-work. In one very base sample, which will be further noted, it was not possible to adhere to this rule because the metal was too hard.
In making such small melts it is practically impossible to adhere to any predetermined composition with any degree of closeness.
The first sample sent out was gold about 105 fine in silver and about 10 fine in copper; 71 assays of this sample were made in nine laboratories, with the following results:

The averages obtained in the different laboratories were:

A sample approximately 500 fine in silver, 110 fine in copper, and 50 fine in lead was assayed 64 times in nine laboratories, with the following results :

The averages obtained in the different laboratories were :

Two samples were both about 25 fine in mixed base metals, while one was approximately 360 fine in silver, and the other was about 450 fine in silver. The first sample was assayed 61 times in nine laboratories, with the following results:
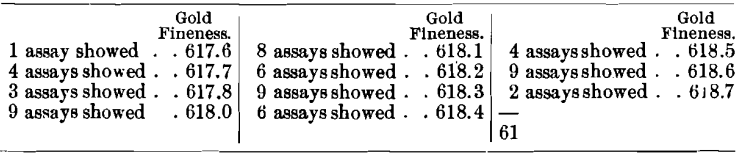
The averages obtained in the different laboratories were:

The second sample was assayed 60 times in nine laboratories, with the following results :
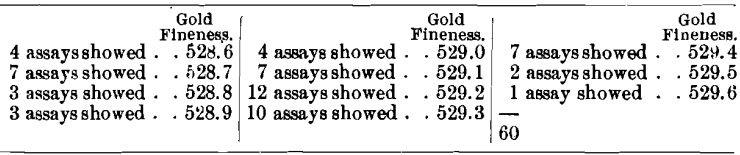
The averages obtained in the different laboratories were :

Having on hand some ferruginous bullion, I attempted to prepare a sample for this work, but experienced considerable difficulty in getting a satisfactory metal, owing to the separation of magnetic globules on solidification. By melting several times with niter I finally obtained a sample that did not show visible segregation, and it must have been close to saturation with iron. It was about 763 fine in gold and 185 fine in silver, so that the entire base metals, including the iron, were only about 52 fine.
This sample was assayed 47 times in nine laboratories, with the following results:

The average results obtained in the different laboratories were:

It having been supposed that much of the difficulty with cyanide gold bars was due to the zinc left in the slimes and going into the bars, a sample was prepared which was nearly 590 fine in gold, about 245 fine in silver, slightly over 130 fine in zinc, and containing a little copper and very little lead.
This sample was assayed 50 times in eight laboratories, with the following results :

The average results obtained in the different laboratories were:

A simple inspection of these results shows very clearly that zinc alone does not materially militate against agreement in the assay-work itself, and if it is the cause of the trouble with cyanide-bars it must be owing to its causing segregation, and thus preventing the proper sampling of the bars by chipping or boring. Other elements may also be active in producing segregation in such bars, either by themselves or through combinations with the zinc or other metals present. A low-grade and very base bar along this line was prepared to run about 100 fine in zinc, 200 fine in copper, and 50 fine in lead. It was about 268 fine in gold and 870 fine in silver. This bar was very hard, and it was impossible to prepare identical samples for the various laboratories. It was simply cut into pieces and a piece sent to each institution.
This sample was assayed 44 times in eight laboratories, and while the difference between the highest and the lowest result is only 1.7 fine, yet the results are scattered all along through the range, and there is only a slight concentration of the results about one point. This is, of course, due in part to the fact that the samples assayed were not identical.
The results obtained were :

It has long been known in a practical way that the presence of arsenic in a gold-bullion prevents any agreement in the assays. Fortunately, however, the presence of arsenic very plainly reveals itself in the melting of the bullion, and when found the melter proceeds to refine the bullion in the pot, and ultimately removes it very completely before the bullion can be accepted.
Three test-samples containing arsenic were prepared, and they yielded most astonishing and interesting results. The first sample was approximately 785 fine in gold, 107.5 fine in silver, 100 fine in copper, and 7.5 fine in arsenic. This is only a small proportion of arsenic, and yet it completely prevented any agreement whatever in the assay-results. This sample was assayed 65 times in ten laboratories. The lowest result obtained was 779.7 fine in gold, and the highest 792.4, with an extreme difference of 12.7 in the fineness. Moreover, there is the utmost divergence in the results as well as no agreement whatever; 30 results were obtained only a single time each, 11 only twice each, 3 only three times each, and only a single result was obtained four times. In only three instances did one laboratory obtain the same result twice.
A sample approximately 675 fine in gold, 200 fine in silver, 100 fine in zinc, 24 fine in lead and copper, and only 1 fine in arsenic, yielded a slightly better set of results, but still very widely divergent. This sample was assayed 62 times in ten laboratories. The lowest result obtained was 671.4 fine in gold, and the highest 681.4, showing an extreme difference of 10 in the fineness; 31 results were obtained a single time only, 10 only twice each, 2 only three times each, and only a single result was obtained five times. In three instances one laboratory obtained the same result twice, and in one case a laboratory obtained the same result three times.
It would appear, however, that the influence of arsenic upon the assaying of high-grade bullion containing only trifling amounts of base metals is far less injurious. While the results on a sample running approximately 865 fine in gold, 130 fine in silver, 1 fine in arsenic, and only 4 fine in other base metals cannot be considered satisfactory, yet they are very much better than those yielded by the other two arsenical bullions. This sample was assayed 53 times in nine laboratories, with the following results:
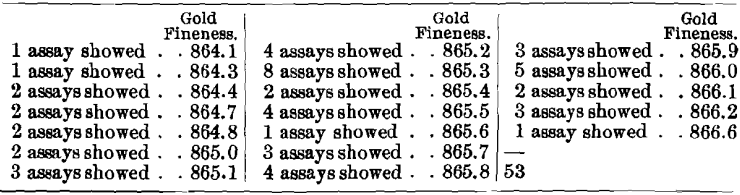
The averages obtained in the different laboratories were:

As in so many other directions, antimony behaves similarly to arsenic in assaying gold-bullion, but its influence is not so pronounced. A sample of bullion approximately 723 fine in gold, 245 fine in silver, 1 fine in antimony, and 31 fine in mixed base metals, copper, lead, zinc, was assayed 46 times in nine laboratories. The lowest assay obtained was 721.3, and the highest 725.1, showing a range of 3.8 in the fineness. However, 24 of the results, or just over a half, ranged from 722.8 to 723.9 fine, and outside of this range only two results were obtained more than a single time.
Finally, some of our gold coin was melted up and assayed 107 times on identical samples in five laboratories, with the following results:

The actual average of this sample is 899.879 fine in gold.
With these results as a basis, the investigation of the subject is being continued with the hope of ascertaining the causes of the variations shown and improving the agreement in the results attained. It is, for instance, well known that gold cornets are not pure gold. They always carry some silver, and I have never failed to find copper in them when tested for with great care. On several occasions I have found lead present on testing the silver nitrate solution from parting a large number of cornets at one time in a platinum basket. The amounts of these base metals present in the cornets are, of course, quite small, and their influence is corrected by the proof-assay, in the same way that it corrects for the silver left in the cornets. I am, however, carrying on a series of quantitative determinations of base metals present in gold cornets, the results of which I hope to publish at some future date.
