Laboratory Assay Equipment
Proper assay equipment is an integral part of every complete ore dressing laboratory and also will find practical use in many industrial and chemical laboratories. No less important are the benefits derived by mining companies through maintaining their own assay laboratories. A private assay laboratory allows the company to set up a more complete and regular schedule for mine and mill assays, and results of these assays are available much sooner. Regularity and rapidity of assays gives closer mill control as milling procedures may be adjusted to fit quality of mill feed or meet smelter requirements.
Assaying for research assay laboratories, commercial assay laboratories or mine assay laboratories is divided into two general classes: fire assays for precious metals such as gold and silver, and wet, or chemical assays for other metals such as copper, lead, zinc, iron, insoluble, etc. Laboratory Equipment includes units for every phase of assaying and recommendations can be made to suit your particular requirements. Many of the necessary units for establishing any desired size of assay laboratory are described in detail in this section. “Assay Laboratory Equipment for Mines,” recommending generally equipment needed and assay laboratory layout, available free on request.
An assay laboratory is usually an essential facility for an operating mine and mill. The extent of the assay laboratory and its equipment depends on the type and nature of the operation. Requirements range from the minimum needed for gold-silver assays to include those necessary for chemical assays of metals, such as copper, iron, lead, zinc and related determinations for the control of the mine and mill operation. The needs and benefits from prompt assay returns in mine development work and in the control of milling operations more than offset the assay laboratory expense.
 The decision on the type of assay laboratory, its equipment and its location when assaying for extensive prospecting, for mine examinations or for a mine in its development stages depends on the number of samples, the difficulties in sample handling and transportation and location of assay facilities. Such field work normally requires a large number of samples and immediate assay returns are of importance. It is often an advantage to have sample results when determining the extent of sampling necessary. Knowledge of sample results can reduce the number of trips into an area in case the assay reports indicate re-sampling or additional sampling is required.
The decision on the type of assay laboratory, its equipment and its location when assaying for extensive prospecting, for mine examinations or for a mine in its development stages depends on the number of samples, the difficulties in sample handling and transportation and location of assay facilities. Such field work normally requires a large number of samples and immediate assay returns are of importance. It is often an advantage to have sample results when determining the extent of sampling necessary. Knowledge of sample results can reduce the number of trips into an area in case the assay reports indicate re-sampling or additional sampling is required.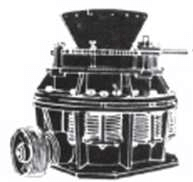
When many samples must be assayed or where the sampling is to be done in remote areas, transporting samples may be both difficult and expensive. The problem in such cases is whether the samples should be sent out or assay equipment brought to the site. In many cases where field sampling is to be done and where the expense and difficulties of setting up a field assay laboratory are great, consideration should be given to equipment for sample reduction and preparation in the field to give lower costs for sample handling and transportation.
It is often of benefit to have samples prepared ready for assaying when given to commercial assayers to avoid any question of careless work in sample preparation and also to provide check samples. Equipment for sample preparation is shown in Group I, “Bucking Room Equipment.” Where electric power is not available the crushing and pulverizing units can be arranged with gasoline engine drives and a gasoline camp stove can be used for drying samples.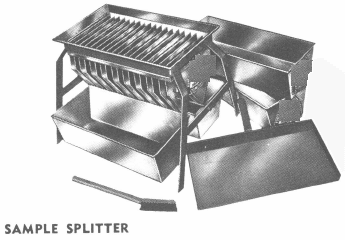
Depending on individual requirements of the company and the nature of the ore to be assayed, samples should normally be reduced in the field to 20 pounds or less. Ores of uniform grade should be pulverized to at least 40 mesh, preferably to 100-150 mesh with quantity cut down to a weight of 100 to 150 grams which is adequate for most assay purposes. Gold-silver ores containing metallics or low grade ores, spotty in nature, may require larger samples of 3 to 5 pounds crushed to about 20 mesh for proper assay procedure where it is necessary to separate metallics by screening or through amalgamation methods.
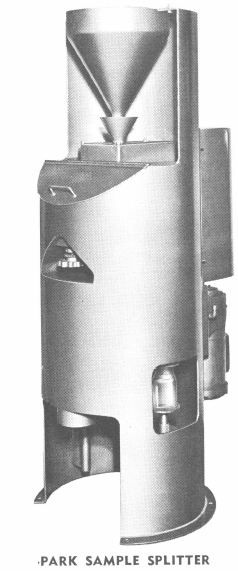
Accuracy of sample splitting is of utmost importance. The patented PARK Sample Splitter has been developed specifically to meet the needs of quickly securing small, representative samples from larger lots.
 In the selection of equipment for an assay laboratory a gasoline, oil or electric assay muffle type furnace may be used. Gasoline furnaces usually cost more to operate but save time due to rapid heating and ease of control. They are considered best for smaller laboratories, or in prospecting and mine examination field work where power is not available. Assayers often prefer a gasoline fired combination furnace having one compartment where crucibles for fusion are in direct contact with the burning fuel and a small muffle for cupellation. Oil fired furnaces are commonly used in mine assay laboratories due to low cost of operation and first cost considerations. Electric assay furnaces are ideal for assaying since the temperature required for various operations can be easily and closely controlled. The installed cost of an electric furnace is about double the cost of other types.
In the selection of equipment for an assay laboratory a gasoline, oil or electric assay muffle type furnace may be used. Gasoline furnaces usually cost more to operate but save time due to rapid heating and ease of control. They are considered best for smaller laboratories, or in prospecting and mine examination field work where power is not available. Assayers often prefer a gasoline fired combination furnace having one compartment where crucibles for fusion are in direct contact with the burning fuel and a small muffle for cupellation. Oil fired furnaces are commonly used in mine assay laboratories due to low cost of operation and first cost considerations. Electric assay furnaces are ideal for assaying since the temperature required for various operations can be easily and closely controlled. The installed cost of an electric furnace is about double the cost of other types.
Normally four types of balances are used in assaying. Harvard Trip type Balances are satisfactory for weighing assay charges or agents and for general use. A low cost pulp balance of about 200 gram capacity with sensitivity of 1.0 milligram is suitable for weighing assay pulp charges. A Button balance for silver need not have high accuracy and one having a sensitivity of 1/300 mg. will meet most requirements. A balance with a sensitivity of 1/500 mg. should be used for gold determinations, and where a large number of gold beads are to be weighed a fast balance of best quality should be used. A balance with milligram weight carriers will aid in the speed of determinations. Button balances should be mounted on concrete piers set into the ground and separate from floors to eliminate vibration from surrounding operations. Often it is necessary to locate the balance room at some distance from the area where crushers and other equipment is operated to avoid transmitted vibrations. Balance rooms should be well insulated and without outside windows to avoid temperature changes as much as possible. Adequate fluorescent lighting is recommended to provide proper light intensity during weighing operations.
 An ample hood should be provided over hot plates especially where wet or chemical assay work or where cyanide solution assays are involved. The hood equipped with an exhaust fan can be built of plywood or sheet metal lined with asbestos board for three feet or more above deck level. Gasoline, gas (natural or bottled) or electric hot plates are available depending on location requirements.
An ample hood should be provided over hot plates especially where wet or chemical assay work or where cyanide solution assays are involved. The hood equipped with an exhaust fan can be built of plywood or sheet metal lined with asbestos board for three feet or more above deck level. Gasoline, gas (natural or bottled) or electric hot plates are available depending on location requirements.
The equipment and supply items listed in Group I, outline items believed essential for sample preparation and in Group II those adequate for 500-750 gold-silver determinations. Group III lists equipment and supply items sufficient for the quantitative determinations of most common metals in ores and mill products. Certain additions or changes may be required in all groups to meet individual and special needs. The lists outline the most common items usually required and will serve as a check list for the selection of items for an assay laboratory.
|
Group I. Bucking Room Equipment |
||
| 2¼” x 3½”Jaw Crusher | Rubber rolling cloth, 36″x 36″ | 24—Sample pans, 8″x 12″ |
| No. 7 Pulverizer | Rubber rolling cloth, 18″x 18″ | 300—Sample envelopes, 4″x 6″ |
| Buck Board and Muller | 1—10″ spatula | 1—Set sieves, 20, 100, 150 mesh |
| Park Sample Splitter | 1—4″ paint brush | 1—Harvard Type Laboratory Scale |
| Riffle Type Sample Splitter | 1—12″ bench brush | 1—Laboratory Drying Oven |
| 8″x 10″ | 1—Hand bellows | 1—Sieve Shaker |
|
Group II. Equipment for Gold andSilver Assays |
||
| 1—Muffle furnace | 2—Sets 12-hole slag molds | 4—Cartons (96 ea.) crucibles |
| 1—Cupel machine | 1—6″ spatula | 1—Carton (320)—2½” scorifiers |
| 1—1/100 Silver Button Balance | 1—Flux scoop | 10 lbs. flour |
| 1—1/500 Gold Button Balance | 1—Pair asbestos gloves | 25 lbs. test lead |
| 1—Assay Pulp Balance | 2—16-hole parting cup trays | 100 lbs. Litharge |
| 1—Set Assay ton weights | 1—Pair button pliers | 150 lbs. Soda Ash |
| 1—Set gram weight, 1/10-100 gr. | 1—Button brush | 160 lbs. Granulated borax |
| 1—Platform scale,50 lb. cap. | 12—Camel hair brushes | 20 lbs. niter |
| 1—Pair 36″ crucible tongs | 1—1″ brush | 5 lbs. Lead foil |
| 1—Pair 36″ cupel tongs | 36—No. 0 Parting cups | 100 lbs. Bone ash |
| 2—Pair 8″ slagging forceps | 6—600 ml beakers | 1 oz. Silver foil |
| 1—Steel sheet-slagging table top | 6—400 ml beakers | 12—5″ roasting dishes |
| 1—Sheet 3/8″ asbestos | 6—250 ml beakers | 5—5 pt. bottles nitric acid |
| 1—6″x6″ slagging anvil | 6—3 in. casseroles | 4—5 pt. bottles hydrochloric acid |
| 1—2½”x2½” button anvil | 2—1000 ml wash bottles | 1 doz. stirring rods |
| 1—16 oz. slagging hammer | 1—Water still | 5 lbs. Lead acetate Tech. |
| 1—3 oz. hammer | 1—Electric hotplate, 8″x 10″ | 1 lb. Zinc dust |
|
Group III. Equipment for Wet Assays |
||
| 5 doz. each—250, 400, 600 ml beakers | 12—Rubber tips, “policemen” | 1 lb. potassium dichromate—CP |
| 1 doz. 500 ml Erlenmeyer flasks | 1—Pair breaker tongs | 1 lb. potassium permanganate—CP |
| 1 doz. 250 ml Erlenmeyer flasks | 1—Pair 12″ crucible tongs | 1 oz. Di-phenyl-amine |
| 6—1000 ml wash bottles | 2 lbs. assorted rubber stoppers | 1 oz. Phenolphthalein |
| 2—Wash bottle fittings | 1—Set cork borers | 1 oz. methyl orange |
| 12—Reagent bottles, 1 gal. and glass stoppers | 12—Pads wax spot paper | 1 oz. potassium ferri-cyanide—CP |
| 12—500 ml reagent bottles | 3—Ring stands with 6 assorted rings | 1 lb. stannous chloride—CP |
| 6—50 ml reagent bottles | 3—Fischer burette clamps | 1 lb. mercuric chloride—CP |
| 2—500 ml volumetric flasks | 1—0-200° C. Thermometer | 1 lb. ammonium oxalate—CP |
| 2—100 ml volumetric flasks | 1—Bottle-Litmus paper | 1 lb. sodium ammonium phosphate—CP |
| 1 each—10, 25, 50, 100 1000 ml
graduated cylinders |
1—Sieve Shaker | 5 lbs. ammonium acetate—CP |
| 1 each—1, 5, 10, 25, 50, 100 ml pipettes | 1—Set 8″ Tyler Test Sieves
¼” through 200 mesh |
1 lb. potassium chlorate—CP |
| 6—50 ml burettes | 2—Funnel Racks | 1 lb. sodium hydroxide—CP |
| 5 doz. each 3, 3½, and 4 inch water glasses | 10—5 pt. bottles, sulfuric acid | 1 lb. sodium carbonate—CP |
| 20 ft. ¼” I.D. glass tubing | 10—5 pt. bottles, hydrochloric acid | 1 lb. potassium carbonate—CP |
| 25 ft. 3/16″ I.D. gum rubber tubing | 10—5 pt. bottles, nitric acid | 1 lb. zinc oxide—CP |
| 6—30 ml iron crucibles | 10—5 pt. bottles, ammonia | 1 lb. sodium acid sulfate—CP |
| 6—30 ml nickel crucibles | 2—5 pt. bottles, acetic acid | 1 lb. sodium peroxide—CP |
| 24—Fire clay annealing cups | 1—5 pt. bottle, perchloric acid | 1 lb. sodium fluoride—CP |
| 36—65 mm pyrex funnels | 2 lbs. hydrofluoric acid | 1 lb. bromine—CP |
| 500—Reeves No. 711 filter papers | 4 oz. CP copper foil | 1 lb. ferric-chloride—CP |
| 500—Wathman No. 40—or Munktel | 1 lb. 20 mesh granulated zinc—CP | 1 oz. silver nitrate—CP |
| No. 0 filter papers | 1 oz. arsenious oxide—CP | 1 lb. barium chloride—CP |
| 1—Box gummed labels | 5 lbs. potassium iodide—USP | 1 lb. iron sulfide-sticks |
| 1—Water still, 2 gal. capacity | ¼ lb. iodine—CP | 1 lb. sodium sulfide—CP |
| 1—Hot plate, gas or electric | ¼ lb. soluble starch | 1 lb. ferrous ammonium sulfate—CP |
| 12—3 inch casseroles | 5 lbs. hypo photographic grade | 1 lb. tartaric acid—CP |
| 1—Pulp balance | 1 lb. sodium thio sulfate—CP | ¼ lb. oxalic acid—CP |
| 1—Analytical balance | 5 lbs. ammonium acetate—CP | 1 lb. sodium bicarbonate—CP |
| 3—Pinch cocks | 1 oz. tannic acid | 10 lb. test lead—CP |
| 3—Hose clamps | 1 lb. ammonium molybdate-CP | 1—H2S generator |
| 2 doz. glass stirring rods | 5 lbs. ammonium chloride—CP | |
| 1 lb. potassium ferro cyanide—CP | ||
| 1 oz. uranium acetate—CP | ||
Assay Laboratory Equipment
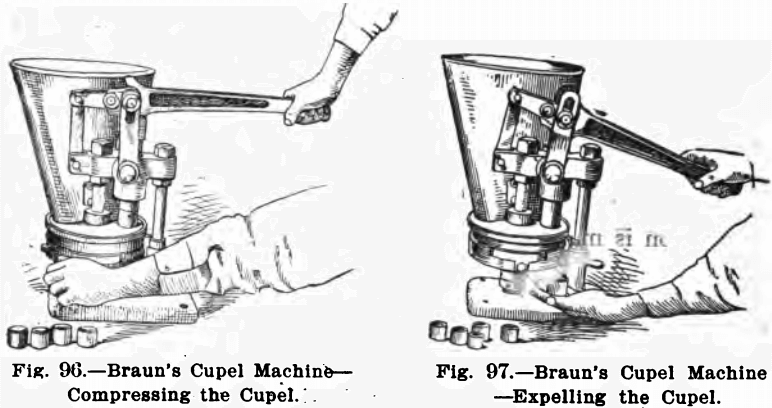
The rest of the equipment of the assay laboratory has much of it been described elsewhere. If the cupels are made by the assayer, a machine for this purpose will be needed. Figs. 95, 96 and 97 show the one made by F. W. Braun & Co., Los Angeles, California.
This machine consists of a hopper to hold the moistened bone ash, and a removable disc with a number of holes which are automatically filled and brought into position under the plunger. To operate, the bone ash is properly moistened and placed in the hopper. In this latter is a small wheel with a rubber rim that keeps the material stirred up and fills the moulds. The lever handle is then raised and the filled mould brought beneath the plunger by means of the handle on the lower disc. See Fig. 95. The downward motion of the lever handle compresses the cupel (see Fig. 96) and by pulling the disc handle in a reverse direction to that formerly given it, the opening in the lower disc is brought beneath the cupel, and further pressure on the lever handle brings a new system of levers into action, expelling the cupel; which may be caught in the hand (see Fig. 97). An automatic attachment stops the discs at the proper points, and an adjusting device is arranged for giving different degrees of compression.
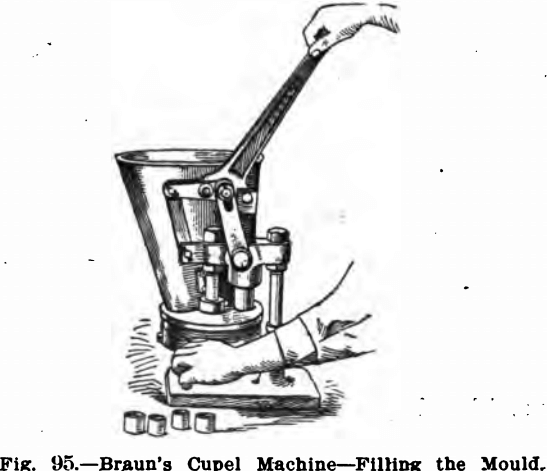
Cupels of five various sizes and depths may be made by using interchangeable discs and dies, which are easily adjusted to the machine.
Its list price is $37.50 fitted for making one size cupels. Dies and discs for other sizes are $10.00 per size.
The bone-ash, which can be purchased in bulk, is moistened with sufficient of a strong solution of carbonate of potash in warm water to make it of about the consistency of damp sand. It should not be pasty, but should show the finger prints, and adhere well together when squeezed in the hand. The cupels should be dried slowly to avoid cracking.
For removing the cupels, scorifiers and crucibles from the furnace, tongs will be needed. These are made in a variety of forms which are illustrated in catalogues of assay supplies. For lifting crucibles, when the operator is above them a pair of “basket” tongs are best suited, while for removing them from a muffle a pair of double bent tongs are best. Cupped lip, or lap lipped cupel tongs grip the cupel most firmly; the former will allow the moving of the cupel from the back to the front of the furnace, and when the muffle is full of cupels. Both the cup and lap-lipped tongs grip the cupel much tighter than the plain lipped. Judson has devised tongs for use with both cupels and scorifiers
which are convenient. They are described in Brown’s Manual of Assaying and listed by Eimer & Amend.
Scorification moulds are also used into which the contents of scorifiers are poured in order that they may cool quickly. They are made of iron and are illustrated in all catalogues of assay appliances.
A muffle scraper is useful for scraping out the muffle after a spill. This consists of a piece of 1/16-inch sheet iron, 6 x 6 inches, bent at right angles, one inch from the end and riveted to an iron rod ¼ inch in diameter and 3 ft. long.
Source: This article is a reproduction of an excerpt of “In the Public Domain” documents held in 911Metallurgy Corp’s private library.
THE ASSAY LABORATORY—For fire-work a room should be set aside, and for convenience it should be situated near the balance room and the chemical laboratory. Gas and water must be laid on if possible, and provision must be made for lighting by day and night (if necessary).
The modern assay laboratory frequently errs on the side of over-equipment. The assayer many times in his after-career has to work without gas and many other luxuries found in large laboratories. It would be well, then, for the student to remember that a ‘ coke- ’ or ‘ coal ’-fired muffle, besides being suitable for cupellation, can be readily adapted to the needs of the chemist. Incinerations, silicate fusions, etc. can with care be accurately performed in the muffle.
Again, a small blast kerosene stove, costing from ten to twenty shillings, can be surmounted by an iron plate or sand bath suitable for boiling, evaporation, etc.
The student may, without unduly interfering with his work, spend some time in exercising his wits on the construction of apparatus. Some of the finest chemical research has been carried through with home-made apparatus ; and he should remember that here, as in many other cases, more depends on the skill of the operator than on an elaborate equipment.
No attempt will be made to furnish detailed plans, but a few general points may be noted:—
Floor; preferably of concrete. Roof; slate or iron (painted), and ventilated by a ‘lantern.’ Walls; preferably of brick. Benches and Lockers; of wood, and placed round the walls. Furnaces ; in the centre of the laboratory.
Fume cupboards, gas, water, grinding appliances, rolls, etc. etc., may be fitted where most convenient.
Besides the laboratory proper, a special room must be set aside for the finer balances. The tables on which these are placed must be of solid construction, and designed to reduce vibration to a minimum. Provision must also be made against dust and fumes from the assay and chemical laboratories.
FITTINGS, FURNACES
Two types of furnaces are required for the efficient performance of the work of the fire-assayer; these are, melting or wind furnaces and muffle furnaces. Many varieties of design are on the market, much depending on the nature of the fuel used, which may be either gaseous, liquid, or solid.
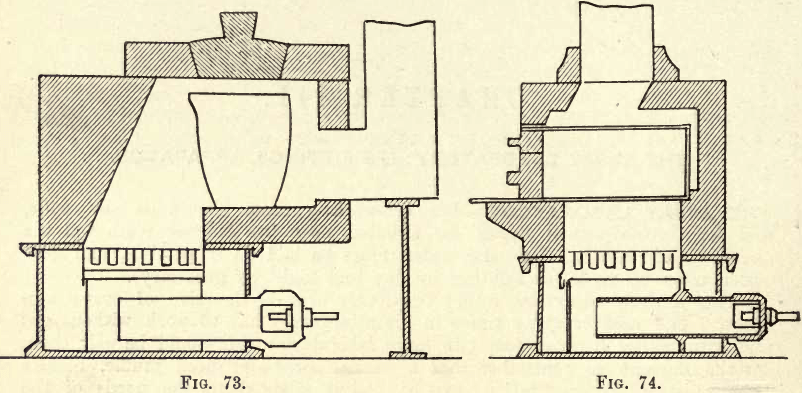
Furnaces for Gaseous Fuel.—These furnaces are designed to consume ordinary illuminating gas, and are simply large and modified bunsen burners. They give good results if carefully handled and regularly cleaned, but can only be recommended where gas is cheap; and as the assayer can but rarely obtain gas at a low figure, the student should, at least at the outset, confine his attention to some other form of furnace. Figures 73 and 74 represent two common forms of Gas Melting and Muffle Furnaces respectively.
Furnaces for Liquid Fuels.—During the past ten years this type of furnace has been brought to great perfection, and portable outfits for melting and cupellation can now be had at a reasonable figure. The outfit consists of three parts,—the oil-reservoir and pump, the burner, and the furnace proper. In out of the way places, where coal or coke is unobtainable or very expen-
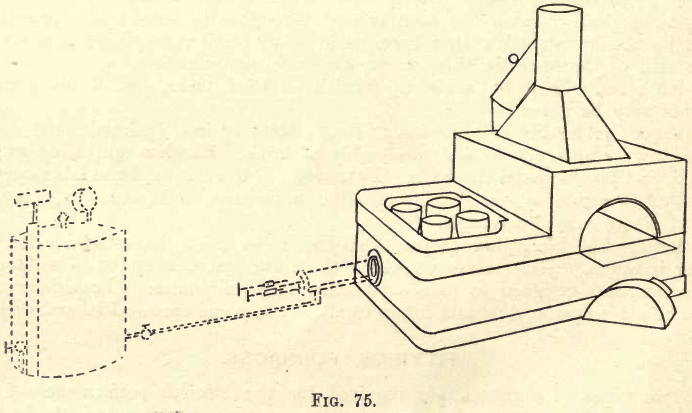
sive, this outfit is very serviceable, as the ‘oil’ or ‘gasoline’ can easily be transported, the volume of oil required being much smaller than that of coal or coke for equal heating effect. Very many varieties of these furnaces are obtainable, some of them displaying much ingenuity. Figure 75 shows a combined Melting and Muffle Furnace.
If gasoline be used as fuel, special care must be taken in following out the directions given regarding the storage and use of this dangerous liquid.
The makers generally supply full instructions regarding the setting up and operation of the furnace.
Furnaces for Solid Fuels.—The fuel used may be coal, coke, or charcoal; generally coke or coal. For the ‘ wind ’ furnace coke is the most suitable fuel, though for the ‘muffle’ furnace, coal has certain advantages over coke; the muffle may be ‘ underfed,’ the fuel being fed underneath and not on the top, thus prolonging the life of the muffle; any free-burning coal may be used, one cwt. of coal running an 8″ x 14” muffle for eight hours. With ordinary prices a saving in fuel as compared with coke results. The fire is quickly started, and the temperature is readily controlled.
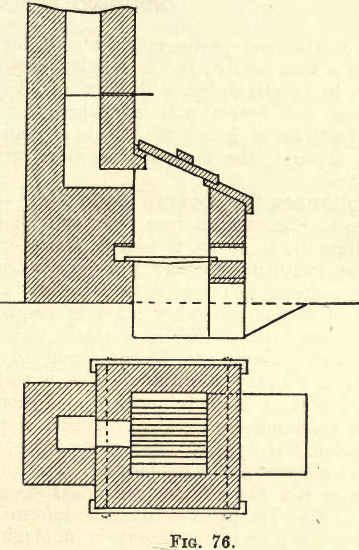
The melting furnace may be constructed of red brick, with a lining of firebrick. It should be provided with movable fire bars, a cast iron top, and a damper to regulate the temperature. Figure 76 shows a convenient design.
The muffle furnace will vary in design according to the nature of the fuel used. In some of the later designs of coal-fired muffle furnaces, two muffles are employed, the one above the other, the upper being used for cupellation and the lower for scorification. Figure 77 shows a furnace designed for the use of coke, and figure 78 a design for a free-burning coal (preferably bituminous).
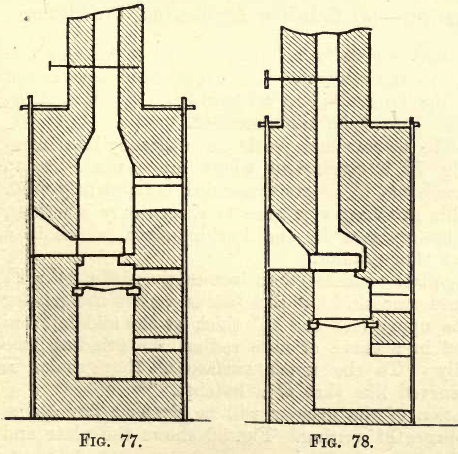
Fire-brick may be used in the construction of these furnaces, though red brick answers well if supported by angle iron and straps. A damper to regulate the temperature will be found very convenient, though not absolutely necessary.
Where proper furnaces were not available, assays have been conducted successfully in an ordinary kitchen range, or on a blacksmith’s forge, with the aid of a small flower-pot for a muffle. For such impromptu appliances the wit and resources of the assayer must be depended on, and in the course of his work the student will have the opportunity of noting where he may replace, partially at least, the appliances of the laboratory by those of everyday use.
GRINDING APPLIANCES.
The mortar and pestle may be used for reducing an ore from the coarse state to a fine powder, but in a laboratory where any considerable amount of work is to be attempted, a division of labour must be adopted by providing appliances for coarse and appliances for fine reduction; by ‘ coarse ’ is meant particles of 1/8 inch to ½ inch in diameter; by ‘fine’ is meant particles, passing through the meshes of a sieve with, say, 20 to 120 holes to the linear inch.
Appliances for Coarse Reduction.—For pieces of ore 9 to 12 inches or more in diameter an iron plate or slab about 36″ x 36″ x 4″ (cast iron); a ‘spalling’ ring about 2 feet in diameter and 4 inches deep and a heavy sledge or knapping hammer will be convenient. By this means the lump may be reduced to pieces 2 to 3 inches in diameter. For further reduction some form of fine pulveriser must be employed.
If ‘power’ be available, a small rock-breaker driven from shafting by a belt and pulleys can be employed to advantage. Here, as in other reducing machinery, a point of vital importance is that the machine be so designed that it may be quickly and thoroughly cleaned. Many otherwise efficient machines are deficient in this respect, and cannot therefore be recommended. If ‘power’ be not available, the same type of machine can be obtained adapted for hand-power. These machines can readily be adjusted to prepare the ore for fine pulverisation on the bucking-plate, or by other means. Fig. 79 shows a convenient form of pestle and mortar.
Where expensive machinery is unobtainable the prospector’s ‘dolly’ can, with a little ingenuity, be adapted to the needs of the assay office.
Apparatus for Fine Reduction—(a) Grinding Appliances; (b) Sieves,
For coarse reduction sieves may generally be dispensed with, but for fine reduction they are indispensable to the student. The experienced assayer can with care reduce a sample on the bucking-plate without the aid of a sieve, but to the beginner this practice cannot be recommended.
(a) Grinding Appliances.—The mortar and pestle have already been mentioned. The form shown in fig. 79 is convenient where much work has to be done. The short pestle, though not to be recommended, is frequently used. It may be safely stated that this grinding appliance is exceedingly laborious, and wherever possible should be replaced by the bucking-plate, which is a cleaner and much more efficient ‘fine-grinder.’
The simplest form of bucking-plate consists of an iron casting 18″ x 24″ x 1″, the upper face of which is planed smooth. On this face is worked the grinder of cast iron, about 4″ x 6″ on the upper face and 1½” thick at the middle, from which, on both sides, it falls off in a curve of wide radius, the grinding face being planed true longitudinally. To the upper surface of the grinder is attached a handle, preferably curved like that of a hatchet.

No description of the operation of the grinder will be given; a few hours’ practice will teach more than pages of writing. Fig. 80 shows the plate and grinder.
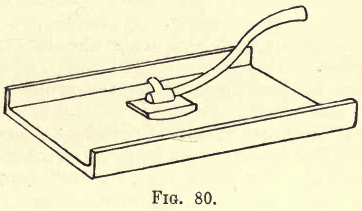
The form shown is simple, and if placed overlapping the edge of the table or other support by about 2 inches the powdered ore is easily brushed off into a scoop or on a sheet of paper. The two longer edges of the plate are often provided with raised flanges to confine the ore. Some makers pivot the plate at the two sides so that it may be tilted for cleaning, but this seems to be an unnecessary refinement. A radical modification of the plate and grinder has recently been placed on the market by an American firm, and
combines the grinder and sieve. The pressure between the grinder and plate is adjusted by simple spiral springs, whilst an ingenious design of the grinder provides a regular feed of ore. The principle of the machine seems good—the feed being regular, the ore being discharged as soon as fine enough, and
the pressure permitting of easy adjustment.
(b) Sieves.—These are generally designated as 20, 30, 40 . . . 100, etc., according to the number of meshes per linear inch, a 20 sieve having 20 holes per linear inch, that is 400 holes per square inch, an
80 sieve having 80 holes per linear inch, or 6400 holes per square inch, and so on. A convenient diameter of sieve is nine inches. It is important when selecting a sieve to choose one that can be readily inspected and cleaned. The most suitable material for the wires is brass; for the body, wood or iron. For
general purposes the following sizes will be useful:— 20, 40, 60, 80, 100.
These sieves may be had in box sets provided with a lid and receiver, thus retaining the dust. The student will find that, when dealing with an ore which clogs the meshes, the operation of sifting is much hastened by placing in the sieve along with the ore two or three small copper coins ; if copper be harmful, discs of some other metal may be employed. The student is reminded finally that he must be absolutely certain of the cleanliness of all reducing and sifting apparatus before proceeding to crush a sample of ore.
GENERAL FITTINGS, APPARATUS, ETC.
The following list of headings, though by no means complete, includes the most important of the smaller pieces of apparatus necessary in the assay laboratory.
Crucibles.—These are made of various materials, and are known as “ Clay,” “ Graphite,” “ French Clay,” “ Hessian,” etc.
“Clay” Crucibles.—Those are best suited for the assays of gold, silver, lead, and tin ores. In the selection of a crucible for a particular assay, the nature of the charge must be taken into consideration : for a basic charge a basic crucible should be chosen, and vice versa. The student will note that when fusing certain charges in these crucibles it is necessary that some substance, such as silica, be added to protect the crucible. Experience will prove to him that with some ores a crucible may serve for three or four fusions, whilst with other ores it may barely serve for one. The cause of this may be discovered by the exercise of a little reasoning, based on the student’s general knowledge of chemistry. Figures 81 and 82 show the round and triangular forms of these crucibles. The round form is perhaps the better, and convenient sizes for the student’s work are those marked D, E, F, G,—D being the smallest and G the largest of the four mentioned. For ordinary purposes a good crucible should be infusible at a bright red
heat : test a chip for half an hour in a strong fire ; the edges should not be rounded. It should also stand sudden changes of temperature : test by sudden heating and cooling. It should also be not too permeable: test by filling with water, and comparing the rate of permeability with that of a good crucible.
Circular lids are provided for the round crucibles, and triangular lids for the triangular form.
French Clay or French Crucibles.—These are more expensive than the ordinary “Clay” variety, and are excellent for the fusion of charges which can be poured. They are manufactured from a mixture of Paris clay and fine sand, and are frequently termed ‘ fluxing-pots.’
Both the “French” and the ordinary “Clay” crucibles may be obtained in a special form suitable for muffle fusions (Colorado crucibles).
The Hessian Crucible is manufactured from a mixture of three parts German clay and one part sand.
The Graphite Crucible in composition consists approximately of a mixture of one to seven parts of clay with three to ten parts of graphite. This crucible will be found serviceable for the fusion of certain metals and alloys.
Strong oxidisers must be added sparingly, if at all, to charges in these crucibles.
Many other kinds and shapes of crucible will be found described in the various text-books on assaying.
Roasting Dishes.—Figure 83 shows in section the form most used. A convenient diameter is from 8 to
12 cm., according to the amount of ore to be roasted. They are usually made from the same clay as crucibles. For roasting quantities greater than 30 grams, iron dishes on top of the wind furnace, if carefully used, are satisfactory.
Scorifiers.—Figure 84 shows in section a form commonly used. The material here, again is a refractory clay. For general work the most convenient diameters are 5, 7, and 10 cm., but for small tests such as the student may wish to perform, 2.5 cm. scorifiers are suitable, taking up little room in the muffle.
It will be noticed that the bottom of the scorifier is thickened. Even with this, ores will be encountered occasionally that corrode completely through between the sides and base.
Muffles.—These are made either of refractory clay or of a mixture of clay and graphite. Those made from clay alone, with care, will be found to answer well. Figure 85 shows the shape commonly adopted.
The “Colorado” muffle is specially designed to receive crucibles, and in section resembles a D lying on its side.
Cupel Moulds and Cupels.—Cupels known as “French Cupels” may be obtained ready for use in boxes of 100 or 144. The student, however, should make his own cupels; in some cases he may even have to prepare bone ash, though this can usually be obtained sufficiently pure.
Figure 86 shows the French cupel, and figure 87 an ordinary form. It is advisable that the upper inner edge of the cupel should be rounded as in a, fig. 88, and not sharp as at b.
This prevents portions of a large button from catching on the sides. To manufacture cupels, moulds are
necessary, and convenient diameters will be 1.8 cm. (for bullion), 2.5 and 3 cm. (for ores). Where a large
number of cupels is daily required, it is advisable to procure a small machine; some of the machines recently designed in America rapidly turn out excellent cupels. Full instructions for the operation of these machines are supplied by the makers.

To make cupels, the mould is thoroughly cleaned; a few pounds of good bone ash are placed in a large tin basin, and water is gently sprinkled over the bone ash, which is at the same time continually stirred. This is continued till the bone ash on being squeezed firmly in the hand just adheres in a lump. The general tendency of beginners is to add too much water, which produces, on drying, a cupel hard as a brick. On rubbing the edge of a dry cupel it should just crumble slightly. Experience alone will teach the right proportion of moisture. The moistened bone ash is placed (not packed) into the mould, the piston is inserted, and several sharp blows are struck with a hammer or mallet. The cupel is then carefully removed and set aside in a warm place to dry. Several weeks should elapse before the cupel is used.
Some assayers employ a solution of ‘‘ pearl ash ” in water, others stale beer to moisten the ash. Other forms of moulds, such as those used at the Royal Mint, are described in advanced text-books.
Furnace and other Tools. — A set of tongs is necessary for handling crucibles, scorifiers, cupels, etc. in the furnaces. Convenient forms are shown

in the following sketches. Fig. 89 shows crucible tongs. The handles of these should be at least .6 metre long (preferably .8 metre).

Figs. 90 and 91 show scorifier and cupel tongs, the handles of which, for comfort, should be at least .8 metre long.
Pokers, scrapers for cleaning out muffles, stirrers for roasting dishes, shovels for coke and coal, cold chisels, etc. etc. are required, besides other odds and ends that want of space forbids mentioning.
Two anvils will be necessary—a block of cast iron (an old die will do) or a small blacksmith’s anvil, and a smaller anvil for line work. This fine anvil must have a smooth polished face. Fig. 92 shows a convenient form.
Two hammers are necessary, one for rough work such as breaking slag, coke, etc., the other for fine work
such as flattening beads, etc. Fig. 93 shows convenient shapes. The small light hammers sometimes
supplied for this purpose should be avoided.
Moulds. — Ingot and slag moulds will also be required in addition to the foregoing.
Besides these articles there are many conveniences, such as cutting pliers, cupel trays, forceps, etc. etc., which the assayer will procure as occasion demands. Complete lists of requisites will be found in the text-books on Assaying.
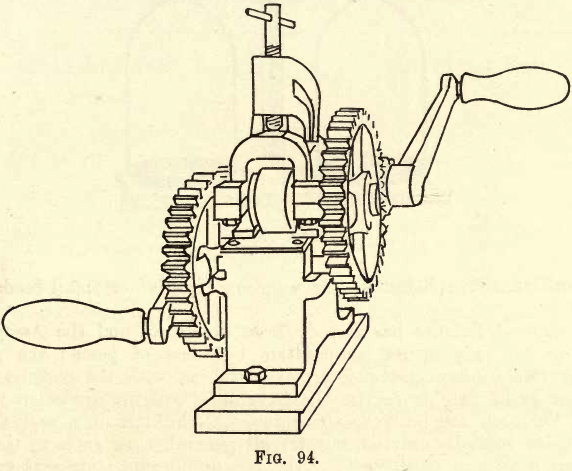
If the assay of gold and silver bullion is to be undertaken, a set of rolls must be added to the equipment. If much of this work is to be done it is advisable that a good set of rolls be procured at the outset. Those with the adjustment of the pressure regulated by one screw are preferable. Fig. 94 shows the “ Braun ” cornet rolls, which have positive feed, delivery and feed plates, and other improvements of use to the assayer.
Chemical Apparatus. — A certain amount of chemical apparatus is required in connection with fire-work. The following list includes the more important requirements in this direction:— Porcelain capsules, royal Berlin (2.5 c.m. diameter) filter funnels, stand, beakers, filter paper, retort stand, bunsen or other burners, wash bottle, etc. ; also a small set of blowpipe apparatus, and the necessary reagents.
BALANCES, WEIGHTS, ETC.
In fire-assaying it will be found convenient to use three balances of varying ranges and degrees of accuracy.
(a) Balances for coarse weighing.—These are not absolutely essential, but will be found convenient for weighing out quantities of several pounds at one weighing. A range of one ounce to ten or twenty pounds will answer well. This balance may be conveniently replaced by ‘ spring ’ scales, which, however, should be checked now and then against standard weights.
(b) Ore Balances of some accuracy. — These are generally termed ‘ pulp scales,’ and should be sensitive to one-tenth of a grain (or one centigramme), with a range of 1000 grains to 1/10th grain (or 100 grammes to 1/100th gramme). These balances are sufficiently delicate for weighing quantities of ore from 10 to 1000 grains. The student should note the degree of accuracy here attained ; e.g. if 400 grains of ore be weighed on such a balance, assuming the weights to be correct, then, as the balance is sensitive to 1/10th of a grain, the error introduced cannot be greater than 1/10th in 400, or 1 in 4000. That is, the result of an assay cannot err more than 1/400th on the plus or minus side. Other errors will occur in the various operations, and these the student should as far as possible ascertain. The algebraic sum of these errors gives the total error.
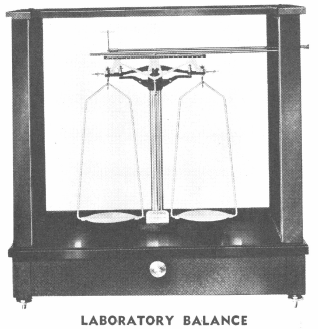
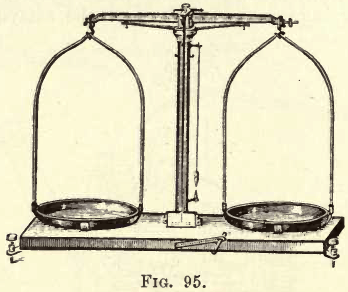
A pair of pulp scales should be provided, with knife edges, levelling; screws, level or plumb-bob and movable pans. Fig. 95 shows a suitable design.
(c) More sensitive Balances, for weighing ‘ prills ’ or small beads of silver and gold.
The Chemical Balance has already been described, and the Assay Balance differs from it chiefly in its comparative lightness of parts; the graduated beam and rider, index and pointer, are read as with the chemical balance, except that grain weights or the ‘ half gramme ’ weights are often used.
Assay Balances are built sensitive to 1/200th milligramme, and even more delicate still if specially ordered, but for all general work such as the student will perform a balance sensitive to 1/20th of a milligramme answers excellently. Using the ‘ten-grain’ set of weights, the balance ‘answers’ to 0.001 grain, and using the ‘half-gramme’ set it ‘answers’ to 0.0001 of ½ gramme, or 1/20th of one milligramme. Thus, if using an ‘ assay ton ’ set of weights based on the 2000 lb. ton, if 1 milligramme represents (see Weights) 1 ounce per ton, the balance is read directly to 1/20th milligramme, that is, to 1 dwt. By the use of special riders or by the methods of weighing previously given the student will see that further accuracy may readily be obtained.
The instructions previously given regarding the care of the balance apply again here. Care and cleanliness are absolutely essential to good work. This last sentence is reiterated for the benefit of the few students in most laboratories who adhere to the practice of placing cupels on the ledge of the balance; or, again, of handling delicate scale-pans with moist fingers. Such students should mend their ways, or be refused entrance to the balance room.
Weights.—In quantitative chemical analysis the ‘gramme’ system has so far been adhered to, and as far as the work of the student in the laboratory is concerned may still be retained; but the fire-assayer finds that in practice he is confronted with ‘long tons,’ ‘round tons,’ and other standards, and that he may have to discard the perfect metrical for a non-metric or a hybrid system. In Great Britain and her Colonies quantities of ore are estimated on the ‘ ton ’ unit; the ton generally equals 2240 lbs. avoirdupois—sometimes, but rarely, 2000 lbs. av. Quantities of gold and silver are estimated on the ‘ troy ounce ’ unit—two conflicting systems, with absolutely no rational basis.
Rationally, the ton should be replaced by the metric unit of 1000 ‘kilos’ ( = 2203 lbs. approx.), the pound being replaced by the ‘kilo’ ( = 2 lbs. 3¼ oz.).
Weights of gold and silver will be represented in grammes and fractions of grammes.
The present disjointed system still holds sway, and the assayer compromises, and makes the best of a bad job by adopting an ‘assay ton’ system. Some prejudice still exists against this method, but even a biased critic will find, on giving it a fair trial, that it is a rational labour-saving device.
According to the unit adopted—whether the ‘long’ ton of 2240 lbs. or the ‘short’ or ‘round’ ton of 2000 lbs.—two systems of ‘assay ton’ weights are in use. The following tabulation shows their relationship :—

If the ore be weighed out in the ordinary way with grain or gramme weights, the contents per ton can only be ascertained from the weight of the bead by a troublesome calculation, or by reference to a set of tables.
The advantage, then, of the Assay Ton system is that the contents per ton are obtained directly, that is, without the aid of calculation or tables. The set of A.T. weights should range from 1/20th to 4 A.Ts.
If A.T. weights are not obtainable, the ordinary grain or gramme weights may be used. These weights will be serviceable for tin and lead ores, but for gold and silver they involve the use of calculation : e.g. 400 grains of ore give a bead of gold weighing 0.012 grains; what are the gold contents per ton? To avoid calculation, a table is drawn up on, say, a 400 grain basis, and giving the contents per ton corresponding to every 1/1000th of a grain from .001 to 1 grain. A similar table may be drawn up for gramme weights, or for any combination of weights (see advanced text-books on Assaying).
For gold bullion it is advisable that a special set of weights be secured, the unit being ‘half a gramme’ = 1000 fine, and ranging with the rider down to .0001 of half a gramme.
A set based on a ‘ten-grain’ unit, and ranging to .001 grain, may be used. Special care should be taken in checking the accuracy (relative) of gold bullion weights. The accuracy of the other weights in relation to the ton used should also be checked by the student before proceeding to weigh ore or ‘ beads.’ The methods of checking have been given in Part II.
In certain cases the assayer may have to report the gold and silver contents of ores in percentages. To do this rapidly a table must be prepared on a 400- grain, 30-gram, or other basis, working out the percentage corresponding to each successive 0.001 grain, or 0.0001 gram, as indicated in the following : —